Problem-solving in the Chemical Industry
LCGC Europe
This month's column looks at some of the problems that the practising mass spectrometrist encounters in the chemical industry today.
In the days before electrospray (ES) and the rapid growth of mass spectrometry as a premier analysis tool in the life sciences sector, petroleum, polymers and environmental samples were the focus of mass spectrometry (MS) and vacuum gas-phase techniques were the most commonly used. This month's column looks at some of the problems that the practicing mass spectrometrist encounters in the chemical industry today. What tools they use to successfully overcome these problems and which tools they look forward to using — or wish they had?
Mass spectrometry (MS) has been a cornerstone technology in the chemical industries, especially in petroleum research and the commercial production of polymers. The American Society for Mass Spectrometry (ASMS) will celebrate its 60th anniversary next year. Although christened under its current name in 1969, it began as "ASTM Committee E-14" in 1952. The work by John Fenn and others that gave us electrospray ionization (ESI) and heralded the modern era where the use of MS for biological analysis predominates, did not start until the 1980s. Before the 1980s gas-phase techniques were the most widely used and techniques were commonly performed in a vacuum as opposed to the atmospheric conditions used today and performed many times as a set experiment using a solids probe, rather than a flowing or chromatographic serial sample introduction.
What interests MS practitioners today? What generates the most problem-solving attention in the chemical industry at the moment? What tools do they use successfully and which ones do they look forward to — or wish they had? I had an opportunity to speak with Colin Moore, Fellow and Technology Leader in Mass Spectrometry at Chemtura Corporation (Middlebury, Connecticut, USA) about the types of analytical problems that his group is asked to solve and what they learned in the process of solving them; this discussion evolved into a short tutorial on chemical industry practice.

A UK native, Moore worked for Shell Research for seven years doing analytical work on agrochemicals and simultaneously became a graduate of the Royal Society of Chemistry. Postgraduate studies at the University of Southampton were followed by three years at Warwick University and a PhD with Professor Keith Jennings. He joined Uniroyal (now Chemtura Corporation) in 1994 as a member of the MS group, becoming manager in 1997 and Research Fellow in 2002. He has 20 publications in peerreviewed journals and 24 posters and presentations at conferences to his credit.
A Simplified Overview
Sample analysis in Moore's laboratory generally falls into one of the following areas:
- Confirmation of the chemical structure of the main components and identification of impurities to help synthetic chemists improve the synthesis.
- Identification of minor components in a product that shouldn't be there (for example, colour bodies).
- Identification of additives in a polymer or oil (for example, antioxidants in engine oil).

Figure 1: GCâMS response for an oil sample (upper is a methanolic extract; lower is the oil sample dissolved in methylene chloride). (Courtesy of Colin Moore, Chemtura Corporation.)
For highly pure samples, analysis by nuclear magnetic resonance (NMR) spectroscopy is probably the best way to obtain detailed structural information and therefore most of the samples that mass spectrometrists are asked to analyse are mixtures. The analyst's first decision, therefore, is to determine which separation technique will be used: gas chromatography (GC), liquid chromatography (LC), gel permeation chromatography (GPC), solid-phase microextraction (SPME) or some type of liquid–liquid extraction (LLE). For example, if an engine oil sample in methylene chloride is shaken with methanol then the phenolic and aminic antioxidants will be concentrated in the methanol. GC–MS analysis of the methanol extract makes it easy to identify the major antioxidants in the oils (Figures 1 and 2).
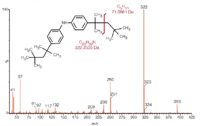
Figure 2: EI spectrum of peak at 13.82 min in the methanol extract shown in Figure 1. (Courtesy of Colin Moore, Chemtura Corporation.)
An interesting aspect of identifying unknowns that, according to Moore, "I've not seen in the literature," stems from making use of both electron ionization (EI) and ESI spectra of an unknown, since the fragmentation patterns can be "complementary" (see the EI and ESI spectra in Figure 3).
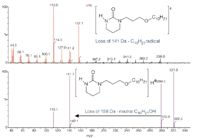
Figure 3: The complementary diagnostic nature of EI and ESI spectra of the same compound. (Courtesy of Colin Moore, Chemtura Corporation.)
In the EI spectrum the first major loss is a C10 alkyl radical whereas in the ESI spectrum it is loss of the C10 alcohol. For those interested in a quick overview on mass spectra a recent column was devoted to that topic1 that, in addition, highlights James Little's insights from his experiences solving problems at Eastman Chemical in Tennessee, USA.
A significant difference in analytical practice between the pharmaceutical and specialty chemical industries is the level of dependence of the latter on GC–MS. Today the pharmaceutical world favours LC–MS. For those interested in the aspects of how LC–MS became what we think of as open access in the pharmaceutical world, I chronicled the insights of a few practitioners on how LC–MS transitioned from a relatively obscure novelty to become the beneficial tool we know today.2 A recent article also examined the ongoing need for development in MS ionization and related technologies to support work in areas where "electrospray just isn't enough" to do the job.3 According to a survey among practitioners in various disciplines, those identified with the chemical materials industry arguably rely on GC three times more than LC. A quick look around Moore's laboratory supports the observation.
One issue that all laboratories need to address is productivity. Two of Moore's GC–MS systems, an Agilent 5970 (Agilent Technologies, Palo Alto, California, USA) and a Waters GCT (Waters Corporation, Milford, Massachusetts, USA), are fitted with two GC columns in the injection port using a two-hole ferrule. One column is connected to the mass spectrometer and the other to an ancillary detection system such as flame ionization detection (FID). As Moore explained "As soon as you tell somebody what an unknown peak is in a GC–MS trace, the next question is usually, how much [of it] is there? Collecting the FID data in parallel with the GC–MS data allows us to give our customers semi-quantitative data as well as MS identification in a single experiment." Such a practice is analogous to LC–MS data acquisition with an in-line UV detector.
Moore's future interests include installing a nitrogen–phosphorus detection (NPD) system, as well as FID, on one GC system because many of the antioxidants that Chemtura makes are amines. Amines as a class are often easily ionized by ESI. Yet in some cases the analytes are just not amenable to the technique. Insufficient polarity, the inability to capture a proton, or excessive volatility that precludes transport and separation by condensed phase means in an LC could all contribute to ESI failure. Great strides have occurred recently to increase the sensitivity, resolution and overall usefulness of LC–ESI-MS instruments. Current techniques — such as atmospheric pressure gas chromatography (APGC) and atmospheric solids analysis probes (ASAP) — which are analogous to the vacuum solids probes used for years in GC–MS, are viable without sacrificing performance, which was not the case years ago.3,4
Data Handling
The ability of software-driven applications to amass increasingly refined data streams has unleashed a data handling problem that crosses into all practices and disciplines. So much so that handling complex data has become a recurring workshop topic at the Conference on Small Molecule Science (CoSMoS) in recent years (www.CoSMoScience.org).
Moore's laboratory uses MassLynx mass spectrometry software (Waters Corporation) to process its MS data. For GC–MS data, workers export the files using the NetCDF converter option in ChemStation software in the three Agilent systems (Agilent Technologies) and then convert the files to the MassLynx format using the Waters DBridge program. NetCDF does not produce a file for the FID system that can be read by MassLynx, and therefore processing the FID trace has to be done using ChemStation. On the GCT system the FID trace is recorded as analogue data by MassLynx that can be processed with the MS data. The retention time differences between the two traces can be time aligned in the chromatogram plots (the peaks come out earlier in the MS data because the vacuum system of the MS increases the helium flow-rate in the column connected to the mass spectrometer).
Understanding the Problem and Designing the Experiment
As an analytical problem, identifying colour bodies in polymers is neither trivial nor obvious. In a 2005 paper,5 Moore shows there is often more than one way to solve a problem using mass spectrometry. Analysis by LC–MS with an inline photodiode-array (PDA) detector, is probably the best way to identify a new colour body. Techniques like time-of-flight secondary ionization mass spectrometry (TOF-SIMS) can be used to quickly confirm that the colour body on fresh samples is the same as on previously determined samples.
Moore's 2005 work detailed the analysis of a yellow discolouration on the surface of a compounded ethylene–propylene–diene monomer (EPDM) rubber sample. Surface discolouration of a polymer can result from various phenomena, including contamination, component migration, oxidation and other chemical reactions. Components rising to the surface can give rise to "bloom," a process in which one component of a polymer mixture (usually not a polymer) undergoes phase separation and migration to an external surface of the mixture, according to the IUPAC definition. Protective waxes can be beneficial, but thiazoles (mercaptobenzothiazole) leading to discolouration of the product are undesirable. For example, oxidation of antioxidants can form colour bodies (that is, phenolic antioxidants can form quinone methides).6 The first step in identifying a colour body is to separate it from the polymer, often by washing the surface with a suitable solvent.
The washings result in a complex mixture of the colour bodies, additives and other surface contaminants. Identification of the coloured components requires further separation of the mixture, analysis of the separated components, and the ability to ascertain which of the components are coloured. The combination of LC–MS–MS with an inline PDA detector is able to do the complete analysis in a single experiment. Moore recalls in 1994 when he joined Uniroyal Chemical, identifying a colour body often involved pooling fractions from multiple LC runs to acquire enough material for the particle beam LC–MS system (an early 1990s rather short-lived technique that, although not very sensitive, produced EI spectra from typical LC-amenable analytes not volatile enough for GC–MS). The much greater sensitivity of ESI sources and TOF mass spectrometers has made the process much quicker and easier, illustrating a central point in mixture analysis: the importance of matching the separation technique with the sensitivity of the final analysis technique.
Matching aspects of the analytical technique is not as simple as it sounds. When light reflects off a coloured substance, the reflected light has the complementary colour to the wavelength or wavelengths absorbed. Yellow light covers the wavelength range 570–585 nm, but the complementary colour to yellow is indigo over the range 420–430 nm. So when processing the LC–MS data, Moore looked for a component with strong absorbtion over that range of wavelengths. He found one component that yielded the UV and LC–MS spectra shown in Figure 4. Note that the colour body is the neutral Cu(II) dibutyl dithiocarbamate, but for it to be detected by the LC–MS system it must be oxidized in the electrospray process to the positively charged Cu(III) compound. A quick review of spectral colour properties can be found at www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/Spectrpy/UV-Vis/spectrum.htm.

Figure 4: Mass spectrum of the colour body (inset) and its UV spectrum. (Courtesy of Colin Moore, Chemtura Corporation.)
Colour bodies are often polar molecules, which means that they are easy to ionize in an ESI source. For some time now, as the instruments' capabilities improved, accurate mass MS has been recognized as an efficient means of enhancing separation of closely related chemical species. As Moore points out, when "the electrospray spectrum contains few (if any) fragment ions, identifying unknowns requires that either an LC–MS–MS spectrum is acquired and/or exact mass measurements7 are performed to get the elemental formula of the pseudo-molecular ion." An inline UV detector is a useful adjunct that is often overlooked in LC–MS. Here the colour of the offending samples, of course, indicates distinct chromophoric benefits. Components separated by the high performance liquid chromatography (HPLC) column absorb at the appropriate wavelength to give the observed discolouration with the added advantage of well-characterized algorithms used by PDA detectors to distinguish differences in homologous components on the samples that the unaided eye cannot.
Moore noted another frequently overlooked element in the analytical chemist's trade that bears mentioning: Structure elucidation is made much easier if the analyst has a thorough knowledge of the sample chemistry and its history.
He also points out that "imaging mass spectrometry is a powerful technique for mapping the concentration of a compound on the surface of a matrix." The technique applies in many fields, including the analysis of inorganic materials, polymers and biological materials. An early publication discusses TOF-SIMS analysis in which a TOF system measures secondary ions produced by bombarding a surface with high-energy particles.8 TOF-SIMS has been used to detect light stabilizers9 and antioxidants10 on the surface of a polymer as well as to characterize the bulk polymer.11
In the few years since Moore published his work, a number of techniques operating by various mechanisms on or near the surface of a material (as opposed to techniques requiring analytes of interest be in solution — desorption electrospray ionization [DESI], direct analysis in real time [DART], ASAP and a few others) have been examined in some detail in this column.4,12,13
DESI can be used in combination with chemical reactions to improve the selectivity and sensitivity of the analysis. Moore's studies with Keith Jennings and attending presentations by Graham Cooks and John Beynon that emphasized the use of chemical reactions in MS encouraged him to attempt using novel chemical ionization (CI) reagent gases to help solve problems. "Many antioxidants are alkylated aromatic amines and therefore a paper by Buchanan14 was of great interest", he says. As shown by the spectra in Figure 5, the technique not only gives the number of aminic protons in the molecular ion, but it also helps identify fragment ions. The m/z = 106 ion in the EI spectrum becomes m/z = 108 in the CI data because of the NH2 group.

Figure 5: Deuterated (ND3) CI and EI spectrum of 4,4'-methylenedianiline. (Courtesy of Colin Moore, Chemtura Corporation.)
Moore has also updated a method first reported by Morgan and colleagues15 for analysing zinc dialkyldithiophosphate (ZDDP) in engine oils. Engine oils are complex mixtures of base oils and performance enhancing multifunctional additives, like ZDDP. They are excellent antiwear agents and effective oxidation and corrosion inhibitors (Figure 6).
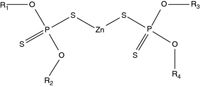
Figure 6: Potential alkyl group positions. (Courtesy of Colin Moore, Chemtura Corporation.)
The original work used negative ion CI to produce chloride ion adducts of the oil without any prior separation. Moore has used an atmospheric pressure chemical ionization (APCI) source and a mobile phase containing methylene chloride to give similar results.

Figure 7: Chloride ion APCI spectrum of an engine oil sample dissolved in methylene chloride. (Courtesy of Colin Moore, Chemtura Corporation.)
Note that the mass spectrum in Figure 7 yields two complementary pieces of information about the ZDDP sample and gives the molecular weight of any phenolic antioxidants in the oil. The phenolic antioxidant present in the oil is evident by the response at m/z = 389 (M–H ion) and at m/z = (M+Cl ion).

Table 1: Understanding the comparative diagnostic value of the ZDDP spectrum*.
The chloride adduct pseudomolecular ion [ZnL2Cl]– permits calculating the total number of carbon atoms in the four alkyl groups (R1+R2+R3+R4). The ligand ions, L–, tell us if the ZDDP was prepared by blending individual ZDDPs or was produced using a mixture of alcohols. Table 1 illustrates how we can deduce that a mixture of C4 and C6 alcohols were used to prepare the ZDDP.
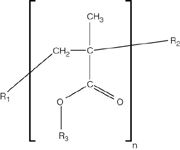
Figure 8: Generic structure for PAMAs. (Courtesy of Colin Moore, Chemtura Corporation.)
Though effective for determining carbon chain length of the R groups, the chloride adduction technique does not show whether the chains are linear or branched. However, collision-induced dissociation (CID)–ion mobility spectrometry (IMS)–CID data may hold the key to solve that puzzle.16

Figure 9: GPC trace for oil sample. 99.5% of the sample yields an Mp of 564 and 0.5% having an Mp of 8630 (Mp, molecular weight, as reported by WatersGPC). (Courtesy of Colin Moore and John Mannello, Chemtura Corporation.)
If the chromatographic conditions are not ideal for interfacing with MS or if other analytical techniques are going to be used to assist in the identification, then LC fraction collection may be the best methodology. Polyalkylmethacrylates (PAMAs) are used as viscosity modifiers in oils (Figure 8). Moore has used fraction collection from a GPC system (Figure 9), then pyrolysis GC–MS (Figure 10) and IR analysis to identify PAMAs. If R1 and R2 are likely either H or methyl and R3 is one of a mixture of alkanes, pyrolysis of this type of polymer gives two series of fragment ions: alkenes and alkyl methacrylates. Thus if R3 is C12 then one gets dodec-1-ene and dodecyl methacrylate (Figure 11).
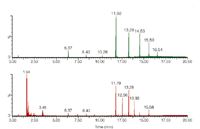
Figure 10: Pyrolysis GCâMS at 550 °C of a PAMA standard (top) and the high mass component from the oil. (Courtesy of Colin Moore, Chemtura Corporation.)
Future Developments
Moore visited Graham Cooks at Purdue to try using a DESI source to detect the colour body.17 Simply spraying the yellow polymer with acetonitrile indeed gave a small signal for the Cu dibutyl dithiocarbamate. Nevertheless, the signal was enhanced when the oxidizing agent I2 was added to the DESI spray solvent.
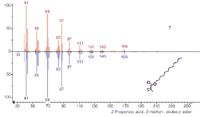
Figure 11: Acquired EI spectrum (top) and best library match for the peak at 11.79 min found in the oil extract (Figure 10). (Courtesy of Colin Moore, Chemtura Corporation.)
An extension of the thermal investigations coming back into favour may, in the not-too-distant future, provide yet another chapter for these studies by combining thermal MS capabilities with the surface information derived by atomic force microscopy (AFM) (Figure 12).
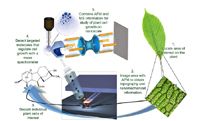
Figure 12: Nanoscale physical and chemical imaging of plant growth regulators using proximal probe thermal desorption MS. (Courtesy of Olga Ovchinnikova and Gary Van Berkel, Organic and Biological Mass Spectrometry Group, Chemical Sciences Division, Oak Ridge National Laboratory.)
AFM uses a sharp-tipped probe, often only 2 μm long and less than 100 Å in diameter, located on the free end of a cantilever, which is brought close to a sample surface. Forces between the tip and the sample surface cause the cantilever to deflect. The deflection of the tip is measured as it is scanned or changes position relative to the sample, generating a surface topographic map. Most commonly tip deflection is a result of interatomic (van der Waals) forces. A good reference for those interested in the topic can be found at http://invsee.asu.edu/nmodules/spmmod/.
Coupling AFM with MS has given rise to an interest termed "molecular cartography" by Gary Van Berkel (Oak Ridge National Laboratory, Oak Ridge, Tennessee, USA). Their work was presented at the 2010 Conference on Small Molecule Science (Portland, Oregon, USA) by Olga Ovchinnikova and can be downloaded at www.CoSMoScience.org.18
Ovchinnikova points out that currently available techniques usually "face a trade-off between spatial resolution and chemical information." Combining spatial resolution, using a heated scanning AFM probe to thermally desorb material from a surface, they then draw the sample into either an ESI or APCI source adding chemical information from the sample surface. The authors refer to the technique as atmospheric-pressure hybrid proximal probe topography chemical imaging. The AFM tip plays a dual role being used for the thermal desorption creating ions for MS analysis while obtaining topographic images of that same surface. Initial results demonstrate the viability of this technique for automated chemical interrogation of caffeine thin films with ~250 nm spatial resolution in the thermal desorption process. Lower resolution proximal probe thermal desorption chemical imaging results of different classes of compounds amenable to this technique include explosives, herbicides, pharmaceuticals and dyes. The authors anticipate this analytical tool "will have broad application for determining the nanoscale spatial distribution of target molecules in plant and animal tissue and material junctions".19
Acknowledgments
The wisdom readers may benefit from in this column is often a distillation from many years of endeavour by people like Colin Moore, and as he recognizes "at the end of the day it's people that solve problems and I'm very fortunate to work with a very talented group of people in the Analytical Services department at Chemtura."
"MS: The Practical Art" editor Michael P. Balogh is principal scientist, LC–MS technology development at Waters Corp. (Milford, Massachusetts, USA); an adjunct professor and visiting scientist at Roger Williams University (Bristol, Rhode Island, USA); and a member of LCGC Europe's Editorial Advisory Board. Direct correspondence about this column to "MS: The Practical Art", LCGC Europe, Advanstar Communications, 4A Bridgegate Pavillion, Chester Business Park, Wrexham Road, Chester CH4 9QH, UK or e-mail: amatherson@advanstar.com
References
1. M.P. Balogh, LCGC North America, 8(2), 122 (2010).
2. M.P. Balogh, LCGC North America, 7(6), 480 (2009).
3. M.P. Balogh, LCGC North America, 8(6), 440 (2010).
4. M.P. Balogh, LCGC North America, 5(4), 368 (2007).
5. C. Moore and P. McKeown, J. Am. Soc. Mass Spectrom., 16, 295–301 (2005).
6. J. Pospíšil et al., J. Polym. Degrad. Stab., 77, 531 (2002).
7. M. Maizels and W.L. Budde, Anal. Chem., 73, 5436 (2001).
8. M.L. Pacholski, and N. Winograd, Chem. Rev., 99, 2977 (1999).
9. F. Andrawes et al., Anal. Chem., 70, 3762 (1998).
10. M.J. Walzak et al., Anal. Chem., 71, 1428 (1999).
11. D. Briggs et al., Surf. Interface Anal.,24, 419 (1996).
12. M.P. Balogh, LCGC North America, 24(1), 46 (2006).
13. M.P. Balogh, LCGC North America, 5(12), 1184 (2007).
14. M.V. Buchanan, Anal. Chem., 54(3), 570–574 (1982).
15. R.P. Morgan et al., Int. J. Mass Spectrom. Ion Phys., 46, 309 (1983).
16. C. Moore and A. Alexander, The Identification of Engine Oil Additives Using Chloride Ion Addition IMS-LCMS/MS, presented at the 58th ASMS Conference on Mass Spectrometry and Allied Topics, Salt Lake City, Utah, 2010.
17. M. Nefliu, R.G. Cooks and C. Moore, J. Am. Soc. Mass Spectrom., 17, 1091–1095 (2006).
18. O.S. Ovchinnikova and G.J. Van Berkel, Molecular Cartography: Moving Towards Combined Topographical and Chemical Imaging using AFM and Mass Spectrometry, presented at CoSMoS 2010, Portland, Oregon, September 25, 2010.
19. O.S. Ovchinnikova and G.J. Van Berkel, Molecular Surface Sampling and Chemical Imaging Using Proximal Probe Thermal Desorption/Secondary Ionization Mass Spectrometry, https://external-portal.ornl.gov/doi/abs/10.1021/ac102766w. Publication Date (Web): December 15, 2010 (Article) DOI: 10.1021/ac102766w.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










