Fifty Years of GC Instrumentation
LCGC North America
This month's installment of "Milestones in Chromatography" pays tribute to a true milestone in the evolution of chromatography: the introduction of the first successful GC instruments.
The possibility of gas chromatography (GC) was first mentioned in 1941, in the famous paper by A.J.P. Martin and R.L.M. Synge. When dealing with liquid-liquid partition chromatography, they predicted that
. . . the mobile phase need not be a liquid but may be a vapour. . . . Very refined separations of volatile substances should therefore be possible in a column in which permanent gas is made to flow over gel impregnated with a nonvolatile solvent. (1)

Leslie S. Ettre
However, at that time, nobody picked up this suggestion and it was 10 years until Martin, now with A.T. James, demonstrated the possibility of gas-liquid partition chromatography (2,3). They worked in the biochemical field and demonstrated the application of the technique by separating and quantitatively determining the components of a C1-C12 fatty acid mixture. The importance of GC was recognized almost immediately by petroleum and petrochemical laboratories, which faced the daily challenge of analyzing complex hydrocarbon mixtures. However, the basic difference between liquid chromatography (LC) and GC became apparent: while classical LC could be carried out in the laboratory by practically everybody using the usual laboratory set-ups, GC required components that were not among the standard laboratory equipment of the time. Companies with large research laboratories usually had mechanical shops capable of constructing the required sophisticated systems. Indeed, the first gas chromatographs were built by the major petrochemical laboratories such as Shell or British Petroleum; however, smaller laboratories did not have the means to construct and build their own systems. Therefore, it soon became obvious that the immense potential of GC could only be fully exploited if proper instruments became available.
Birth of an Industry
The years following World War II saw the birth of the scientific instrument industry. The first real "instruments" developed and built were infrared spectrophotometers, but at that time, these were used only in a limited number of laboratories, and their operation and use required special skills. The gas chromatographs represented the first truly automated, complex analytical instruments that did not need specially skilled scientists for their operation and could be used by practically every laboratory. Thus, it is not surprising that the early availability of mass-produced gas chromatographs had a major role in the rapid expansion of the technique and its use. At the same time, this new industrial branch greatly benefited from the growing demand for this instrument. We might even say that some kind of symbiosis existed between GC and the scientific instrument industry: the evolution of the former could not have happened without the involvement of the latter. A number of companies prominent today in the scientific instrumentation field started as small companies founded by some enterprising chemists starting to build gas chromatographs for the analytical chemists.
A couple of British companies (Griffin & George, London, UK, and Metropolitan Vickers Electrical Co., Manchester, UK) tried to provide gas chromatographs soon after the publication of James and Martin's paper. However, they did not have the resources for more fundamental development work, and their instruments were not much different from a self-constructed laboratory set-up. The field soon was taken over by American companies, which gained experience in electronics and optics and in producing high-precision systems to fulfill the need of the Allied military during World War II. The two companies introducing gas chromatographs almost simultaneously in the spring of 1955 (three years after the publication of James and Martin) were the Burrell Corporation (Pittsburgh, Pennsylvania) and the Perkin-Elmer Corporation (Norwalk, Connecticut).
The Burrell Instruments
Burrell Technical Supply Corporation was founded after World War I to supply gas analysis equipment. In 1943, it introduced a system for the determination of the components of natural gas by selective adsorption-desorption. This was the so-called Turner-Burrell Adsorption Fractionator and was based upon the work of Nelson C. Turner (4,5). It was a fairly complicated, floor-standing machine, and its use was very tedious: one analysis took at least 8 h and required a 2-4 L gas sample. In subsequent years, the instrument was simplified somewhat, reducing analysis time to 1-3 h: the result was the so-called Fracton, introduced in 1953 (6).
Both Burrell instruments utilized what we would call today a combination of frontal and displacement analysis. The large gas sample was adsorbed on the column and then it was pushed upward by a combination of a moving heater and a displacer: liquid mercury in the Turner instrument and tetrachloroethylene vapor in the Fracton. During this slow movement, the sample components were more or less separated. Finally, the separated sample fractions were purged by hydrogen gas into a thermal-conductivity detector. As can be seen, neither instrument was very user-friendly and today, the Occupational Safety and Health Administration (OSHA) would certainly stop their use within moments. Can readers imagine a free flow of mercury and tetrachloroethylene vapor in the laboratory?
At the September 1954 National Meeting of the American Chemical Society, H.W. Patton of Tennessee Eastman Co. (Kingsport, Tennessee) presented the first American paper on GC (7). In it, he described a self-constructed system using an adsorption column in the elution chromatography mode, an inert carrier gas and commercially available thermal-conductivity cells as the detector. L.V. Guild of Burrell was present at the meeting, and he realized the possibility of modifying the Fracton into a "real" gas chromatograph by using a carrier gas instead of the displacer. The new instrument, the so-called Kromo-Tog Model K-1 (Figure 1) was introduced in March 1955. It used an unheated charcoal column and had only limited use for the analysis of samples that were gas or vapor at room temperature. A fairly complicated bypass device was used for sample introduction, and there was no possibility to inject liquid samples.

Figure 1: The Burrell Kromo-Top Model K-1 gas chromatograph introduced in March 1955. The cover over the U-shaped column was removed; it was not thermostated and was at room temperature. Gas samples could be introduced with a multivalve inlet system (behind the small door just below the recorder).
In the next few years, Burrell improved this instrument, adding the possibility of column heating (with a wraparound nichrome heating wire) and liquid injection. However, the company did not have the needed R&D resources to keep up with continuous improvements, and thus, in the first part of the 1960s, the production and marketing of gas chromatographs was discontinued.
Perkin-Elmer's Vapor Fractometer
While the Burrell instrument was relatively short lived, the one introduced by Perkin-Elmer almost simultaneously represented the first instrument in a continuous line of gas chromatographs, descendants of which are still manufactured by the Life & Analytical Science Division of PerkinElmer, the successor of the original Perkin-Elmer Instrument Division.
The Perkin-Elmer Corporation was founded in 1937 and established itself during the war as one of the leading manufacturers of precision optics. In mid-1940, the company also became involved in manufacturing infrared (IR) spectrophotometers and through this product line had increasing contacts with leading scientists in the United States and Europe. While visiting Professor Thompson at Oxford University in 1953-1954, Perkin-Elmer's representative heard for the first time about the GC work of C.S.G. Phillips at Oxford and of James and Martin in the laboratories of the British Medical Council in London. Harry H. Hausdorff, then the head of Perkin-Elmer's IR applications laboratory, went to visit them to learn more about the new technique; upon returning home, he gave an enthusiastic report about its possibilities. Based upon these visits and also upon information from E.F. Williams at the central research laboratories of American Cyanamid Co. (Stamford, Connecticut), a breadboard model was constructed by the middle of 1954 (Figure 2), and detailed investigations were carried out on the best instrument design and on the influence of operation parameters on the analytical results. This led to the development of the final version of the instrument that was introduced in May 1955: this was the so-called Model 154 Vapor Fractometer (Figure 3). Besides Hausdorff, two other Perkin-Elmer associates should be mentioned who participated in the development of the instrument: A. Savitsky and E.S. Watson.

Figure 2: The prototype of Perkin-Elmer's gas chromatograph built in 1954. The construction of the air thermostat for the column and the detector block (behind the door on the left side) already was similar to the final version, but sample injection still was through a serum cap into the carrier gas stream, just before the column (small door at the lower left).
The Model 154 became an instant success. This was best demonstrated by the comments of Ralph H. Müller the legendary columnist of Analytical Chemistry who within one month after the introduction of the instrument characterized it in his monthly column as "a splendid example of automatic analysis." Commenting on the chromatograms that were obtained (using a potentiometric recorder), he stated that, "If the reader can tolerate our exuberance, which we hope is contagious, these recordings are a delight to behold." (8).

Figure 3: The final version of the Perkin-Elmer Model 154 Vapor Fractometer, with Harry H. Hausdorff, who had a major role in its development. The door on the left side covers the air thermostat for the U-shaped column mounted on the thermal-conductivity detector block. A flash vaporizer was incorporated into the system with a rubber septum, permitting syringe injection of liquid samples (small circle at the lower left). Starting with the Model 154-B, the potentiometric recorder was housed in a box of the same size as the instrument (see Figure 6).
Three major factors contributed to the success of the Model 154. The first was its versatility and ease of operation. The instrument had most of the features one can find in present-day gas chromatographs. The U-shaped column was placed in an air thermostat capable of temperatures as high as 150 °C (within a year this was upgraded to 225 °C). Column effluent was conducted directly into a heated thermal-conductivity detector, with special construction utilizing thermistor beads. Liquid samples were injected with a syringe through a rubber septum into a flash vaporizer, and the vapors were swept by the carrier gas stream into the column. Within a year, a newly developed rotary-type gas sampling valve was added, permitting reproducible introduction of constant-volume gas samples. To ensure the constancy of the carrier gas flow, the instrument was equipped with the proper controls and monitoring devices.
Perkin-Elmer immediately recognized the importance of the availability of columns with a variety of stationary phases. At introduction, columns containing both adsorption and partition-type packings were available from the company, and within a year their list was extended to 10 different columns. In fact, Perkin-Elmer was the first instrument manufacturer to provide standard columns. The wide range of available columns was particularly important in this period, before the general acceptance of capillary columns: due to the relatively limited efficiency of packed columns, the possibility of choosing the most selective stationary phase was crucial.
The second major factor contributing to the rapid acceptance of the technique was the help Perkin-Elmer gave to the starting chromatographers by providing a simple text explaining the principles of GC and of the selection of operational parameters. We must not forget that in 1955, everyone was a novice and that at that time, no textbooks or general papers were available dealing with the principles of the technique: the 31-page booklet by Hausdorff published in September 1955 (9) was thus of extreme importance. Ralph Müller devoted a special section of one of his editorials to this publication, characterizing it as "a compact and very informative summary of the theory, uses, instrumentation and general practices of gas chromatography." According toMüller:
. . . the instrument manufacturers in this country are doing a splendid and increasingly important job in disseminating scientific and technical information. . . . Analysts are heavily indebted to our more progressive instrument manufacturers who are contributing in this way to the collection of definitive information on the newer methods of analysis. (10)
The third factor in the success of the Model 154 was the company's realization that in the case of a new technique, it is important to show by illustrative examples how typical analytical problems can be solved. Müller in his quoted article (8) included a typical chromatogram showing the separation of a wide-range sample (the famous "eight-component mixture") in 24 min (Figure 4) and the very first advertising of the Model 154 in Analytical Chemistry (11) presented the analysis of C1-C5 hydrocarbons in 23 min (Figure 5). Solving these problems with the then-existing methods would have been possible only by complicated and very tedious manipulations, taking many hours of hard work.
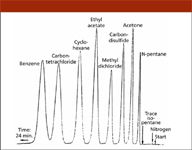
Figure 4: The chromatogram of an eight-component mixture included in Ralph Müllers editorial in the June 1955 issue of Analytical Chemistry, which described the instrument (8).
However, showing some typical analyses would not have been enough; the prospective users expected help in solving their actual analytical problems. Perkin-Elmer already had extensive experience in customer contact in the field of IR spectroscopy and now, it was extending this service to the new field of GC. The company's applications group was continuously in contact with chemists in the widest possible fields, running their samples and helping in establishing the optimum conditions for their analyses. At the same time, through this continuous contact, the company learned about the needs of the practical users which then could be incorporated in the next instrument model. It is safe to say that without this symbiosis between manufacturer and user, without the extensive activities of the Applications Engineering Groups, the meteoric rise of GC would have been impossible.

Figure 5: The chromatogram showing the separation of 12 C1-C5 hydrocarbons in 23 min, used in the first advertising of Perkin-Elmers Model 154 (11). A 2-m long column containing 30% triisobutylene on Celite was used at room temperature. The ad also explained the way for quantitative evaluation of the chromatogram.
In the 1950s and early 1960s, Perkin-Elmer published a widely read quarterly magazine titled Instrument News. It contained descriptions of the newest instrumentation, news, and editorials, as well as technical articles written by both company associates and users of Perkin-Elmer instruments. Some of these articles were very authoritative. For example, I remember one authored by J. Edgar Hoover, the director of the F.B.I., on the use of IR spectroscopy in criminological studies. In essence, this periodical can be considered the precursor of present technical news magazines such as LCGC. An article in the Fall 1955 issue of Instrument News characterized the enthusiastic reception of the Model 154 by saying that
We have a wildcat by the tail in our new Model 154 Vapor Fractometer, a unique instrument based on gas chromatography. Its enthusiastic reception has us breathless, our lab engulfed by requests for application studies and everybody happy. (12)
For more than five years, the Model 154 was the most widely used gas chromatograph (Figure 6). As mentioned, within one year, its upper temperature limit was extended and a gas-sampling valve added (Model 154-B). Then in 1959, the newly developed flame-ionization detector and the possibility of using capillary columns were added to the instrument, first as an accessory (Model 154-C) and the next year, as an integral part of the instrument (Model 154-D). In 1961, a simplified version of the instrument, with a lower upper temperature limit and with only thermal-conductivity detection, also was added for light gas analysis only (Model 154-L). Separate versions of the instrument also were manufactured by Perkin-Elmer's German (Model 116) and English (Models 451 and 452) subsidiaries, and in 1957, a special triple-stage instrument (Model 188) also became available for the analysis of wide-boiling-range samples.

Figure 6: The control laboratory of a petroleum company in 1957, showing eight Model 154s. The operator is standing before a box containing a potentiometric recorder. The recorders for the other instruments are on the lower level of the laboratory bench.
Proliferation of Instruments
Burrell and Perkin-Elmer were soon followed by a number of other companies, both in the United States and overseas, introducing new gas chromatographs. By 1958, five additional companies were in the market with seven additional models, and by 1962, the number of American companies offering gas chromatographs grew to 25. In addition to the instruments of Burrell and Perkin-Elmer, these companies offered 42 different instruments, with various additional features (for example, temperature-programming) (13). This plethora of gas chromatographic instruments greatly contributed to the meteoric rise of the technique (13,14). Starting in 1962, Perkin-Elmer also introduced new, more sophisticated gas chromatographs. However, the Model 154 and its European counterparts continued to be produced until the late 1960s, and many laboratories kept their old instrument in use for decades. Just recently, I have learned of a Model 154-B originally purchased in 1956 by an industrial laboratory in Sweden that remained in constant use for more than 30 years. Then the company donated it to a training facility for hospital technicians where it was used until 2003. (Figure 7) The unparalleled history of this instrument is the best testimony of the foresight of the developers of the Model 154, which started the GC revolution.

Figure 7: A model 154-B gas chromatograph, still in use after 47 years, in the training facilities of Karolinska Institute, Stockholm, Sweden. The operator is Hans Traènker. The photo was taken in 2003.
Acknowledgments
The photograph shown in Figure 7 was obtained from Perkin-Elmer Sverige AB, Upplands Va¨sby. The other figures are from the author's personal collection.
References
(1) A.J.P. Martin and R.L.M. Synge,
Biochem. J.
35
, 1358-1368 (1941).
(2) A.T. James and A.J.P. Martin, Biochem. J. 50, 679-690 (1952).
(3) A.T. James and A.J.P. Martin, Analyst 77, 915-932 (1952).
(4) N.C. Turner, Natl. Petrol. News 35(18), R234-R237 (1943).
(5) N.C. Turner, Petrol. Refiner 22, 140-144 (1943).
(6) L.S. Ettre, Chromatographia 55, 497-504 (2002).
(7) H.W. Patton, J.L. Levis, and W.I. Kaye, 126th National Am. Chem. Soc. Meeting, New York, New York, Sept. 12-17, 1954; Anal. Chem. 17, 170-174 (1955).
(8) R.H. Müller Anal. Chem. 27(6), 33A-36A (1955).
(9) H.H. Hausdorff, Vapor Fractometry (Gas Chromatography): A Powerful New Tool in Chemical Analysis (Perkin-Elmer Corp., Norwalk, Connecticut, 1955), p. 31.
(10) R.H. Müller Anal. Chem. 27(12), 33A-35A (1955).
(11) Anal. Chem. 27(9), 22A (1955).
(12) Instrument News 7(1), Fall 1955.
(13) L.S. Ettre, J. Chromatogr. Sci. 15, 90-110 (1977).
(14) L.S. Ettre, J. Chromatogr. Sci. 40, 458-472 (2002).
Leslie S. Ettre"Milestones in Chromatography" editor Leslie S. Ettre is a research affiliate of the Chemical Engineering department of Yale University and a member of LCGC's editorial advisory board. Direct correspondence about this column to "Milestones in Chromatography," LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail lcgcedit@lcgcmag.com.

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.














