Extracolumn Effects
LCGC North America
When you look at the manufacturer's literature or examine the performance sheet included with a new column, you'll see a list of column specifications, including the column plate number N. For a 5-?m particle size, column N generally will be 80,000 plates/m or more, whereas a 3-?m column will exhibit 100,000 or more theoretical plates. Your first response might be, "Get real!" After all, when real samples are analyzed on typical liquid chromatographic (LC) systems, rarely do we observe plate numbers anywhere near the manufacturer's claim.
When you look at the manufacturer's literature or examine the performance sheet included with a new column, you'll see a list of column specifications, including the column plate number N. For a 5-μm particle size, column N generally will be 80,000 plates/m or more, whereas a 3-μm column will exhibit 100,000 or more theoretical plates. Your first response might be, "Get real!" After all, when real samples are analyzed on typical liquid chromatographic (LC) systems, rarely do we observe plate numbers anywhere near the manufacturer's claim. This is in part due to the nonideal behavior of many sample molecules but also depends upon the system plumbing. Some of this performance degradation is due to extracolumn effects. These comprise any band-broadening effects other than the column: injection volume, detector characteristics, time constants, and plumbing. This month's installment of "LC Troubleshooting" will concentrate on the impact plumbing can have on column performance.

John W. Dolan
Contributions to Peak Width
The peak width observed in a chromatogram (
V
obs
) is the result of several factors as expressed in equation 1:
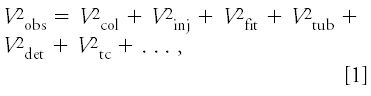
where the observed peak width is a combination of the contributions from the column (Vcol), injection (Vinj), fittings (Vfit), connecting tubing (Vtub), detector cell (Vdet), detector and data system time constants (Vtc), and so forth. Each of the terms is squared in the summation because we must add the variances to get the proper statistical total. The observed peak width can be expressed as volume by multiplying the measured baseline peak width in time units by the flow rate. Each of the contributions to the observed peak in equation 1 is important, as are other factors not listed here. In this discussion, we'll examine only the contribution of connecting tubing - it should be obvious that the other factors can have equal or greater impact under particular circumstances.
Model Conditions
As a basis for this discussion, we'll look at six column configurations that might be encountered or at least considered for routine separations. Two column lengths were selected: 150 mm to represent the most common column length for routine LC-UV methods and 50 mm to represent the shorter columns used for LC-mass spectrometry (MS) or "fast LC" separations. Popular internal diameters for these columns are 4.6, 2.1, and 1.0 mm. Packing particle diameters of 3-5 μm are widely available; I've chosen to use 3-μm particles because they place a little more demand on the system in terms of extracolumn effects. Under ideal conditions (manufacturer's test conditions), these columns will generate 100,000-150,000 plates/m, or
N
â 20,000 for the 150 mm configurations and
N
â 6700 for the 50-mm lengths.
Most of us are worried not about column efficiency, but about resolution and speed. We want to separate the various peaks in our sample and we want to do it fast. For the separation selected here, just two peaks are considered. The first one has a retention factor k of 2.0, which is the recommended minimum by United States Food and Drug Administration (FDA) and International Conference on Harmonization (ICH) guidances for routine analysis. The retention of the second peak is adjusted to generate a resolution of 2.0 under ideal conditions (no connecting tubing). Figure 1 shows a visual comparison of resolution values.
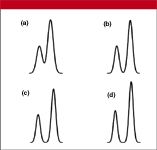
Figure 1: Peak resolution: (a) 1.0, (b) 1.5, (c) 1.8, and (d) 2.0.
I'm not going to go into detail on the calculations, but for those of you who want to explore this topic further, the essential equations are listed below. Rearrange them as necessary, and with the help of an Excel spreadsheet, you can generate the data presented here. Just be careful to watch the units!
The core equations are
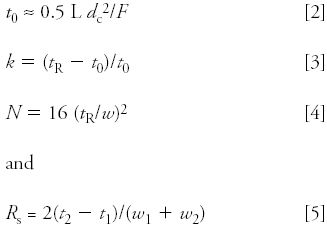
The column dead time t0 (in minutes) can be estimated from the column length L, diameter dc (both in centimeters), and the flow rate F (in milliliters per minute). Equation 3 for the retention factor k can be rearranged to solve for the retention time tR. Equation 4 can be solved for peak width w. Retention times (t1 and t2) and peak widths (w1 and w2) for the two peaks of interest are used to calculate resolution Rs with equation 5.
Those of us who have been using 250 or 150 mm à 4.6 mm columns packed with 5-μm particles probably never have noticed much deterioration of a separation that could be attributed to plumbing. The data of Table I show that 25 cm of 0.007-in. i.d. connecting tubing has no perceptible effect on resolution with 3-μm particles for the 150 mm à 4.6 mm column; the impact on a 5-μm particle column would be less. In fact, even 100 cm of 0.010-in. i.d. tubing only degrades the resolution from 2 to about 1.9 (data not shown) for the 3-μm column. I've often speculated that one of the reasons for the success of LC as an analytical technique is that users can be pretty sloppy about technique and still get good results with the columns typically used for analytical purposes.

Table I: Effect of connecting tubing length and diameter on resolution for various 3-mm column sizes
Let's see what happens when other column configurations are used. Today we're seeing a move toward smaller packing particles, because all other things being equal, they will give narrower, taller peaks, and thus, better detection limits. Additionally, narrower diameter columns reduce solvent consumption. As a rule, I recommend the use of 0.007-in. i.d. tubing to connect the column to the autosampler and detector; 0.005-in. i.d. tubing is more prone to blockage and might not add much to column performance. This is borne out by the data of Table I for the 3-μm, 150-mm column in either 4.6 or 2.1 mm i.d. In each case, 25 cm of 0.007-in. tubing has little or no impact on the separation. Remember that the 25-cm tubing run must be split between the autosampler and column, and the column and detector, but for most LC systems, this is sufficient tubing to connect a 150-mm column.
The 2.1-mm i.d. column has approximately 20% of the cross-sectional area of the 4.6-mm column, so at the same linear velocity of mobile phase, it will use about one fifth of the mobile phase as the larger column and give the same retention times. What happens if a 1.0-mm i.d. column is used? The data in Table I show us that the 25-cm run of 0.007-in. i.d. tubing will cost us approximately 25% of the resolution with the 150 mm à 1.0 mm column. See Figure 1 for a visual illustration of this change. Clearly, the 0.007-in. i.d. tubing is not satisfactory for this application. On the other hand, use of 0.005-in. i.d. tubing will result in less than a 5% loss in resolution. The column volume of the 1.0-mm i.d. column is only 5% of the 4.6-mm column; peak widths will be correspondingly narrower. The second column in Table II lists peak volumes (widths) for the various column configurations. We can see that columns that generate narrower peaks are more sensitive to extracolumn effects such as the contribution by connecting tubing.
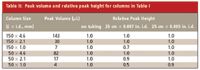
Table II: Peak volume and relative peak height for columns in Table I
What's the Concern?
For conventional separations, the most popular column configurations are the 150 mm à 4.6 and 2.1 mm sizes packed with either 3- or 5-μm particles. For simple separations, many workers like to use shorter 50 mm à 4.6 mm i.d. columns packed with 3-μm particles for what is sometimes termed "fast chromatography." Table I shows us that these short, fat columns are not very sensitive to plumbing. This is because the peak volumes are relatively large (see Table II). For LC-MS applications, the 50 mm à 2.1 mm column configuration is very popular because it gives sufficient resolution for these applications, generates narrow peaks, and uses mobile phase flow rates of 0.2-0.5 mL/min, which work well with most LC-MS interfaces. Table I shows that a loss of approximately 6% in resolution can be expected with 25-cm of 0.007-in. i.d. connecting tubing, but the same length of 0.005-in. i.d. tubing maintains resolution. However, in many cases, the physical configuration of an LC-MS system is such that longer tubing runs are required. One should be careful to keep the tubing length to a minimum, because the separation can degrade even with 0.005-in. tubing if the length is sufficient. For example, 1 m of 0.005-in. i.d. tubing will reduce resolution by approximately 20% (last column of Table I; see Figure 1 for a visual comparison). Even short runs of tubing will compromise resolution with the narrow 50 mm à 1.0 mm columns, as seen in Table I. Half the resolution is lost with 25 cm of 0.007-in. i.d. tubing, and approximately 10% is lost with the same length of 0.005-in. tubing.
Additional Considerations
So we have seen that if we are conservative about the diameter and length of connecting tubing, we should see little loss of resolution for columns with inner diameters of 2.1 mm or greater, but narrower bore columns might not perform as well as we expect. Let's look at a couple of other factors that can be important.
First, let's consider peak height. When limits of quantification or limits of detection are of concern, such as trace analysis in environmental samples, pharmacokinetic assays, or impurities assays, peak height is more important than peak area. For these types of applications, narrow-diameter columns often are selected because they generate taller peaks for the same mass on column. As a first approximation, a chromatographic peak can be considered an isosceles triangle: any decrease in peak width translates proportionally to peak height, because peak area should be constant. Thus, the peak volume data of the second column of Table II show that with constant area, a 2.1-mm i.d. column should generate peaks fivefold taller than their 4.6-mm i.d. counterparts; 1.0-mm i.d. columns would produce peaks 20 times as tall. This assumes that one can load the same mass on each column, which may or may not be true. The mass loadability of a column is proportional to the cross-sectional area. The loadability of a 4.6-mm column is 20-fold more than the 1.0-mm column, so it is likely that the mass of sample injected must be reduced on the smaller column to prevent mass overload. However, if mass overload is not an issue, the remainder of Table II shows the reduction in peak height for various column and tubing combinations for the same mass on column. It can be seen that peak height does not suffer greatly except for the 1.0-mm i.d. columns using 0.007-in. i.d. tubing.
A second concern might be how the added volume affects run time. If the system suitability test requires a minimum resolution of 2.0 and resolution is degraded by adding tubing, one would have to modify the method conditions to increase resolution. For a simple, two-component sample, improving resolution by changing the mobile phase composition might not be too difficult, but with a complex sample, improving the resolution of one peak pair often reduces the resolution of another pair. As a general rule, increasing resolution costs run time. After all, if better resolution could be obtained with shorter run times, it is likely that the method would have been developed to have a shorter run time and better resolution - a win-win situation! Longer run times mean lower throughput, and for any routine method, this will increase the analysis cost per sample.
So far, we've looked only at connecting tubing. Another factor that plays into the observed peak width, according to equation 1, is the injection volume. A rule of thumb for injection volume says that you can inject up to about 15% of the peak volume without a noticeable decrease in resolution when the mobile phase is used as the injection solvent. Consultation of the peak volume data of Table II shows that this guideline does not allow for very large injection volumes. For example, the 150 mm à 4.6 mm column would accommodate only a 20-μL injection before peak broadening due to injection effects that can arise. All of the other column configurations shown in Table II would require even smaller injection volumes. Most of us will have a hard time keeping the injection volume this small, especially with the smaller volume columns. Many methods use injection volumes larger than this recommendation. This suggests to me that the injection volume is likely to play a much larger role in the degradation of column performance than the connecting tubing for most applications.
All of the data shown in Tables I and II are for isocratic separations. Gradient runs also are affected by plumbing, but the effects are complicated by a factor called band compression. Injections for gradient methods often are made in a solvent weaker than the mobile phase. This compresses the peak width at the head of the column, effectively eliminating any band broadening created by tubing upstream from the column. This means that the tubing between the column and the detector is important for gradient separations, but the precolumn tubing is of little consequence. Peak widths in isocratic separations, on the other hand, are influenced by all the connecting tubing between the autosampler and the detector.
Conclusions
We started this examination of the influence of connecting tubing on column performance with the implication that tubing was a very important factor. However, the data in Tables I and II show that for 2.1-mm i.d. or larger columns, the influence of connecting tubing is of little importance if we are conservative about the length and diameter of connecting tubing used. Columns packed with 5-μm particles will be influenced even less than the 3-μm particle columns studied here. Peaks with longer retention times will be broader and also less influenced by tubing effects. When 1.0-mm i.d. columns are used, plumbing effects can be much more important, so short lengths of 0.005-in. i.d. tubing should be used. Injection effects are more likely to compromise column performance than tubing selection for most separations.
My recommendations? Don't use 1.0-mm i.d. columns unless you really need them. The 2.1-mm columns provide sufficiently narrow peaks for most work, give a fivefold savings in mobile phase use, and are fairly insensitive to connecting tubing. As a rule, I like to stay away from 0.005-in. i.d. tubing because it is much more prone to blockage than the 0.007-in. tubing, and little performance gain is realized for most applications. If you do use 0.005-in. tubing, be sure to place a 0.5-μm porosity in-line filter just downstream from the autosampler to trap any particulate matter that might block the tubing.
John W. Dolan "LC Troubleshooting" Editor John W. Dolan is Vice-President of BASi Northwest Laboratory of McMinnville, Oregon; a Principal Instructor for LC Resources, Walnut Creek, California; and a member of LCGC's editorial advisory board. Direct correspondence about this column to "LC Troubleshooting," LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail John.Dolan@Bioanalytical.com.
For an ongoing discussion of LC trouble-shooting with John Dolan and other chromatographers, visit the Chromatography Forum discussion group at http://www.chromforum.com.

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).















