HPLC Analysis of Nonvolatile Analytes Using Charged Aerosol Detection
LCGC North America
A new detection method based upon aerosol charging was examined for its applicability and performance with high performance liquid chromatography (HPLC). Our results demonstrate universal detection of nonvolatile analytes with response magnitude that is independent of analyte chemical properties, four orders of magnitude dynamic range, low nanogram, lower limits of detection, and < 2% relative standard deviation response variability. Broad applicability was demonstrated for a range of methods including those using gradient elution, reversed phase, hydrophilic interaction, and ion chromatography; normal and narrow bore column formats; and in combination with other detectors (for example, UV detectors, evaporative light-scattering detectors, and mass spectrometers).
There continues to be strong demand for improvements in sensitivity, selectivity, throughput, qualitative content, and many other performance characteristics of high performance liquid chromatography (HPLC). A major need is for methods that provide both universal detection and quantitative analysis. It is widely recognized that no single HPLC detector is capable of distinguishing all possible analytes from a given chromatographic eluent and the term "universal" often is used to describe detection of a diverse range of analytes.
A primary goal for most analyses that seek universal detection is to obtain a consistent relationship between the magnitude of response and quantity injected for a range of analytes. This "consistency of response factors" allows the use of global mathematical relationships to estimate quantity (for example, use of parent drug response factor to quantify metabolites and degradants). This characteristic is useful in many applications, where it is impractical or impossible to use individual standards to calibrate the response for each analyte such as drug library QC, pharmaceutical impurity testing, complex lipid analyses, and many applications requiring mass balance assessment. Currently, performing such an analysis is difficult with available technologies.
Several techniques are employed for universal detection that are based upon measuring bulk properties or generic attributes among diverse analytes. These techniques include refractive index (RI), low-wavelength UV, evaporative light-scattering detection (ELSD), and chemiluminescent nitrogen detection. These techniques are in contrast to those geared toward selective detection, which typically is based upon measurement of specific properties or reactivity of analytes (for example, fluorescence at a specific wavelength, oxidation at a specific potential, and mass-to-charge ratio [m/z]). It should be noted that for most detectors, selectivity and universality are mutually exclusive. Universal detectors often require very selective sample prepurification and analytical separation techniques for analysis of biological matrices.
Various characteristics of commercially available instruments employed for universal detection have been compared previously (1-3). RI, while widely used, has significant limitations in sensitivity and is not compatible with gradient elution. Low-wavelength UV provides higher sensitivity and improved gradient compatibility. However, detection is limited to chromophores, and response magnitude depends upon molar absorbtivity, which can vary by orders of magnitude even among analogous structures. Although UV detection remains the primary technique for many HPLC analyses, it is unable to see compounds that lack a sufficient UV chromophore such as many underivatized amino acids, carbohydrates, lipids, polymers, surfactants, drug substances, and natural products. ELSD has become widely used alone or to complement absorbance and mass spectrometry (MS) detectors (3). ELSD can see compounds lacking a chromophore provided they have low volatility. Also, ELSD response magnitude often is less dependent on analyte chemical properties than MS or UV. ELSD, however, has significant limitations in precision, sensitivity, dynamic range, and the nature of calibration curves (1,4,5). Chemiluminescent nitrogen detection is a relatively new technology for nitrogen-containing molecules and, while less universal, can detect a broad range of pharmaceutical compounds. It has been reported that this detection technique can provide a more linear response and lower limit of detection (LOD) than ELSD, but with poorer precision, higher maintenance, and no compatibility with nitrogen-containing mobile phases such as those with acetonitrile and triethylamine (1). The demand for improved methods continues to drive novel approaches toward universal HPLC detection such as those involving condensation nucleation light scattering (5) and inductively coupled plasma MS (6).
A new HPLC detection method has been developed based upon charged aerosol detection (CAD). This technique is fundamentally different from that of other detectors and is based upon the coupling of HPLC with widely used electrical aerosol analyzer technology (4,7-10). A similar HPLC detection method, termed aerosol charge detection, was described by Dixon and Peterson (4). The detection principle involves the charging of aerosol particles via corona discharge with subsequent electrometer-based measurement and thus has some commonality with atmospheric-pressure chemical ionization (APCI) MS. However, CAD operates by detecting charged particles that have a selected range of mobility rather than by measuring individual gas-phase ions that are differentiated based upon m/z. Furthermore, previous studies have shown that the signal obtained with electrical aerosol technology depends primarily upon particle size across a wide range and does not depend significantly upon individual analyte properties (9). This more generic principle can thus theoretically provide consistent interanalyte response factors and complement atmospheric pressure ionization MS techniques such as electrospray and APCI.
Described in the following sections is our initial evaluation of this technique. We tested the general applicability of CAD using various HPLC systems and modes of separation and for a diversity of analytes. Particular emphasis was on detection of nonchrompohores and to assess dependence of response upon analyte chemical properties and chromatographic variables. We also evaluated CAD for its use, in parallel, with MS and photodiode-array detection for high-throughput analysis of diverse small-molecule pharmaceutical library compounds using typical methods for quality control and for support of synthesis.
Experimental
A diagram of the CAD device is shown in Figure 1. The detection method involves pneumatic nebulization of HPLC column eluent, exclusion of the largest droplets via an impactor, and ambient T solvent evaporation to form an aerosol of analyte particles. A turbulent jet of positive ions, formed by passing a secondary stream of gas by a corona discharge and through an orifice, is mixed with the opposing analyte aerosol stream (8). In this process, charge is transferred diffusionally to analyte particles. Excess positive ions are trapped by a weak electric field and charged particles impinge on a conductive filter, which transfers the charge to an electrometer for signal transduction.
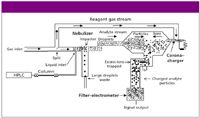
Figure 1: Conceptual diagram of CAD.
Several CAD devices (Corona CAD, ESA Inc., Chelmsford, Massachusetts) were evaluated with a variety of HPLC systems, including Agilent 1100LC (Agilent Technologies, Wilmington, Delaware) and ESA HPLC systems. Nitrogen gas (ESA nitrogen generator or cylinder), regulated at 35 psi was introduced to the detector and the resultant gas flow rate was regulated automatically and monitored by the CAD device. The only parameter that required user input was response range, which typically was set to 100 pA full scale with 1 V analog output to the Agilent ChemStation or EZChrom Elite for ESA chromatography data systems. Initial studies of detection scope and response factors involved flow injection analysis (FIA) of compounds representing a wide diversity of chemical properties. Precision, sensitivity, dynamic range, and nature of calibration curves were evaluated for a variety of compounds and chromatographic conditions and included some comparison with UV and ELSD. Specific conditions are described with results.
Results and Discussion
To study the chemical scope of CAD and dependence of response on analyte nature, we performed FIA of 17 chemically diverse compounds. Results and conditions, summarized in Table I, show that all analytes, except for the volatile compound naphthol, were detected by CAD. Using these conditions, naphthol was detected by UV but did not respond with low-temperature ELSD (data not shown). These results suggest that CAD response depends upon analyte volatility. This is as expected based upon similar observations with other techniques that involve particle detection subsequent to solvent evaporation (for example, ELSD).
Peak area from FIA of a constant mass (10 μg) of each analyte ranged from 1.78-2.34 à 106 units across two devices with 6.44 and 8.31% RSD within each instrument (Table I). Because these analytes represent a wide range of molecular weights, polarity, acid-base characteristics, and included several nonchromophores, these results demonstrate that response magnitude does not depend significantly upon analyte chemical structure or properties. This is consistent with the described independence of electrical aerosol measurement on the nature of analytes (9).
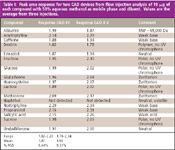
Table I: Peak area response for two CAD devices from flow injection analysis of 10 mg of each compound with 50% aqueous methanol as mobile phase and diluent. Values are the average from three injections.
The detection scope was characterized further for 735 compounds from Millennium Pharmaceuticals' (Cambridge, Massachusetts) small molecule library. Compound molecular masses ranged from 165 to 684 Da and included a wide range of chemical structures and properties from several drug discovery programs. CAD was used in combination with photodiode-array detection, single-quadrupole MS, and ELSD, as shown in Figure 2. Using the described conditions, the same 733 compounds were detected by both CAD and ELSD. The only two compounds that did not respond were those with the lowest MW (165 and 178 Da). This provides further evidence of the dependence of response on analyte volatility and demonstrates that CAD, which utilizes ambient temperature evaporation, has a similar detection scope to low-temperature ELSD.
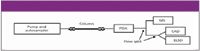
Figure 2: LC-PDA-MS-ELSD-CAD system. Column: 35 mm 3 4.6 mm, 3-mm dp CapCell Pak MG C18 (Shiseido, Tokyo, Japan); binary gradient: 100% solvent A to 100% B in either 5 or 10 min; solvent A: 98:2 (v/v) water-isopropanol and solvent B: 75:25 (v/v) acetonitrile-methanol, each containing 10 mM ammonium acetate; temperature: ambient; flow rate: 1.5 mL/min.
CAD response factors (area/micrograms) were highly variable with >50% RSD for the 733 library compounds detected. Because our FIA results demonstrated that there was no significant dependence of response upon chemical structure, we therefore studied the dependence of response upon analytical conditions. We performed FIA of six compounds of known purity using various mixtures of solvents A and B, as described in Figure 2. The results demonstrate that both ELSD (not shown) and CAD response were dependent upon eluent composition.
Figure 3 shows that CAD response was directly proportional to eluent percentage organic content, and that the magnitude of this effect was very similar for all compounds. These results are consistent with the theory of nebulization, specific droplet size selection (for example, impactor, siphon), and evaporation. This effect, in which higher solvent volatility favors delivery of analyte for subsequent detection, has been observed widely for many techniques that involve solvent evaporation (for example, atmospheric pressure ionization [API]-MS, ELSD) (5). This is further demonstrated in Figures 4 and 5, which show the response obtained for a constant mass of several compounds separated by gradient reversed-phase and hydrophilic interaction chromatography (HILIC) (11), respectively. As shown, peak area either increases or decreases proportionally with retention time as a consequence of a corresponding increase or decrease in percentage organic content during gradientelution.

Figure 3: Peak area CAD response from FIA of seven compounds using three different isocratic conditions showing response proportionality to aqueous-organic eluent composition. Percentage composition refers to solvents A and B described for Figure 2.
As shown in Figures 3-5, the dependence of CAD response upon eluent composition was predictable and uniform among diverse analytes. Based upon these observations, our ongoing efforts are to examine specifically the use of empirical and computational approaches as a means of normalizing CAD response. These approaches will be applied to a broader scope of Millennium Pharmaceutical's compound library (that is, more compounds, greater chemical diversity) to assess the practical capabilities of CAD to provide uniform response factors (that is, accurate quantitation) across numerous analytical runs and in support of several ongoing drug discovery programs.
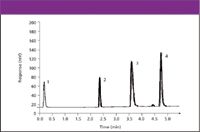
Figure 4: Gradient reversed-phase separation and detection of diverse compounds with CAD. Column, mobile phases, flow rate, and temperature were the same as in Figure 2. Gradient: 0-100% B in 5 min. Sample: 5 mg, 10 replicate injections. Peaks: 1 5 glucose, 2 5 caffeine, 3 5 propranolol, 4 5 chlorpromazine.
The chromatograms in Figures 4 through 8 show several examples of the use of CAD for a variety of separations. This includes its use with several solvents, additives, and conditions including gradient elution (Figures 4 and 5), reversed phase (Figures 4 and 8), hydrophilic interaction (Figures 5 and 6), and ion chromatography (Figure 7). As expected, our data show that CAD has similar requirements for mobile phase volatility as API-MS and ELSD. In addition to that shown, the detection technique also was compatible with many other solvents and modifiers including acetone, methylene chloride, hexane, triethylamine, and trifluoroacetic acid. Figure 8 shows the use of CAD with several HPLC column formats. Using a single nebulizer design and the described conditions, peak width increased and response decreased only when using a column inner diameter less than 2 mm. These results are in accordance with our measurements of an effective detector volume of approximately 12 μL. This demonstrates good compatibility with narrow and normal bore chromatography, and further study is required to address lower volume formats.
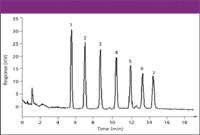
Figure 5: CAD of oligosaccharides using gradient HILIC. Column: 250 mm 3 4.6 mm, 5-mm dp Asahipakm NH2P (Shodex); mobile phase A: acetonitrile; mobile phase B: water; gradient: 70-30% A in 40 min; flow rate: 1.0 mL/min; temperature: 35 ðC; sample: 200 ng. Peaks: 1 5 DP1, 2 5 DP2, 3 5 DP3, 4 5 DP4, 5 5 DP5, 6 5 DP6, 7 5 DP7.
Figure 4 is an overlay of 10 replicate injections in which response variability (percent RSD) was 1.62, 1.75, 1.14, and 1.48 for glucose, caffeine, propranolol, and chlorpromazine, respectively. Variability obtained with ELSD, using these same conditions, was 3.52, 3.61, 2.95, and 2.41 %RSD. for these compounds. This twofold or more improvement in precision over ELSD has been observed consistently in several applications in which we have compared CAD and ELSD. The lower limit of detection for the previously mentioned analytes was estimated using serially diluted standards and determined as the lowest mass injected that yielded a peak-to-peak signal-to-noise ratio of 3 or greater. The limit of detection ranged from 5 to 20 ng for CAD and 100 to 150 for ELSD. Figure 6 provides an example of CAD sensitivity for simple sugars. The 10-fold or better improvement in LOD obtained with CAD has been consistently observed again in several comparison studies with ELSD.

Figure 6: Isocratic HILIC separation and CAD of simple carbohydrates. Column: 250 mm 3 4.6 mm, 5-mm dp Asahipak NH2P (Shodex); mobile phase: 70:30 (v/v) acetonitrile-water; flow rate: 1.0 mL/min; temp: 35 ðC; sample: 10 ng. Peaks: 1 5 fructose, 2 5 glucose, 3 5 sucrose, 4 5 lactose.
Figure 9 shows that a dynamic range of at least four orders of magnitude (typically 10 ng to 100 μg) was obtained with CAD with very similar calibration curves (that is, response factors) for all five phenolic compounds. CAD response was nonlinear and can be represented by y = axb where y = peak response (height or area), x = analyte mass, and b = the exponential response factor (that is, sensitivity). The slope determined from a log-log plot of response versus concentration for these five phenolic compounds ranged from 0.48 to 0.50. This response behavior is in substantial agreement with both theory and empirical data on pneumatic nebulization and aerosol charging (8). Because CAD and low-temperature ELSD use comparable techniques for aerosol formation, the observed improvements in precision, sensitivity, and dynamic range likely result from differences in particle detection technology. It is widely recognized that light-scattering efficiency is dependent upon particle diameter and involves exponential changes between scattering regimes (that is, Rayleigh, Mie, refraction, reflection). This has been shown to underlie the dramatic "drop-off" in response, limited sensitivity, and high response variability often observed with ELSD (4,5). In contrast, the response obtained with electrical aerosol detection technology used with CAD has been shown to have unimodal efficiency over a broad range of particle diameters (4,8). These performance characteristics can provide significant advantages in many applications including those requiring detection of diverse compounds and trace (for example, impurity, metabolite) analyses.
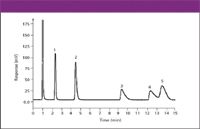
Figure 7: Isocratic ion chromatographic separation and CAD of anions. Column: 100 mm 3 4.1 mm, 10-mm dp PRP-X100 (Hamilton, Reno, Nevada); mobile phase: water with 60 mM ammonium hydroxide and 100 mM formic acid (isocratic); flow rate: 1 mL/min; temperature: ambient. Peaks: 1 5 phosphate, 2 5 chloride, 3 5 bromide, 4 5 nitrate, 5 5 sulfate.
Conclusions
The described studies demonstrate that charged aerosol detection applies to a broad range of HPLC separations and appears to possess a number of very desirable characteristics. These include: universal detection of nonvolatile analytes, response independent of chemical properties, a wide dynamic response range with high sensitivity, good precision for a wide diversity of analytes, and simple and reliable operation. The magnitude of CAD response was found to be proportional to eluent percentage organic content. Additional study is therefore required to determine if the observed predictable nature of this dependency can provide a means of normalizing response for accurate quantitation of unknowns. The applicability of this technique to biological matrices also requires additional study.
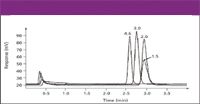
Figure 8: CAD response for a constant mass of 1 mg of caffeine analyzed with 4.6-, 3.0-, 2.0-, and 1.5-mm i.d. column formats. Columns: 75-mm long, 3-mm dp CapCell Pak C18 (Shiseido, Tokyo, Japan); mobile phase: 20% aqueous methanol. Flow rate was scaled proportionally with column volume (for example, 1.2 mL/min for the 4.6-mm i.d. column and 0.128 mL/min for the 1.5-mm i.d. column). Injection volumes were 40, 20, 16, and 8 mL, respectively, for the 4.6-, 3.0-, 2.0-, and 1.5-mm i.d. columns.
Acknowledgments
The authors would like to thank S.L. Kaufman, TSI, Inc. (Shoreview, Minnesota) for his fundamental work with electrical aerosol technology, its initial coupling with liquid-phase techniques, and for his valuable technical insight.

Figure 9: Dynamic response range of CAD.
References
(1) M.C. Allgeier, M.A. Nussbaum, and D.S. Risley,
LCGC
21
(4), 376 (2003).
(2) K. Petritis, C. Elfakir, and M. Dreux, J. Chromatogr., A 961, 9-21 (2002).
(3) D.A. Yurek, D.L. Branch, and M.S. Kuo, J. Comb. Chem. 4, 138-148 (2002).
(4) R.W. Dixon, and D.S. Peterson, Anal. Chem. 74, 2930-2937 (2002).
(5) J.A. Koropchak, L.E. Magnusson, M. Heybroek, S. Sadain, X. Yang, and M.P. Anisimov, Adv. Chromatogr. 40, 275-314 (2000).
(6) P.A. Lawless, J. Aerosol Sci. 27, 191-215 (1996).
(7) A. Medved, F. Dorman, S.L. Kaufman, and A. Pocher, J. Aerosol Sci. 31, S616-S617 (2000).
(8) B.Y.H. Liu and D.Y.H. Pui, J. Aerosol Sci. 6, 249 (1975).
(9) R.C. Flagan, Aerosol Sci. Technol. 28 (1998).
(10) A.J. Alpert, J. Chromatogr. 499, 177-196 (1990).
Paul H. Gamache is director, applications, with ESA, Inc., Chelmsford, Massachusetts.
Ryan S. McCarthy is an applications specialist with ESA, Inc., Chelmsford, Massachusetts.
Scott M. Freeto is applications manager with ESA, Inc., Chelmsford, Massachusetts.
Darwin J. Asa marketing manager with ESA, Inc., Chelmsford, Massachusetts.
Matthew J. Woodcock, is a research associate, Millennium Pharmaceuticals, Cambridge, Massachusetts.
Katherine A. Laws is a research investigator with Millennium Pharmaceuticals, Cambridge, Massachusetts.
Rod O. Cole is director, analytical sciences, with Millennium Pharmaceuticals, Cambridge, Massachusetts.
ESA Inc., Chelmsford, Massachusetts
*Millennium Pharmaceuticals, Inc., Cambridge, Massachusetts
Address correspondence to P.H. Gamache, e-mail pgamache@esainc.com.

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).
Silvia Radenkovic on Building Connections in the Scientific Community
April 11th 2025In the second part of our conversation with Silvia Radenkovic, she shares insights into her involvement in scientific organizations and offers advice for young scientists looking to engage more in scientific organizations.














