Evaluation of Achiral Stationary Phases for Gradient Screening with Supercritical Fluid Chromatography
LCGC North America
Unlike reversed-phase liquid chromatography, SFC lacks a universal stationary phase. Thus, it is important to re-evaluate the default column screening library used with SFC. In this study, three uncommon achiral SFC columns were investigated and compared to three popular stationary phases.
Analytical and preparative supercritical fluid chromatography (SFC) is increasingly implemented beyond chiral separation purposes. In contrast to reversed-phase liquid chromatography, SFC lacks a universal stationary phase, and therefore the outcome of SFC is highly influenced by screening experiments. Consequently, as new stationary phases and drug substances are introduced, it is important to re-evaluate the default column screening library to expand selectivity for the separation of multicomponent reaction mixtures. In this study, the orthogonality, separation performance, and π-π selectivity of three uncommon achiral SFC columns (pyridyl amide, nitro[phenyl], and naphthyl) were investigated and compared to three more popular stationary phases, using pharmaceutically relevant analytes and a reference mixture of 18 polycyclic aromatic hydrocarbons. A high degree of similarity (r > 0.94) was shown for: (1) ethylpyridine, basic, and pyridyl amide phases, and (2) cyano and diol phases. The results illustrate the complementary potential of these column chemistries, particularly naphthyl, for SFC screening workflows.
Supercritical fluid chromatography (SFC) has achieved a dominant role in the pharmaceutical industry as a screening and purification technique for chiral analytes (1–5), and is quickly becoming a powerful technology for achiral separation as well (6–9). Much of the success of SFC is owed to its faster separation speed and lower solvent consumption (10). The reduction in evaporation time during purification is an additional benefit afforded by SFC relative to liquid chromatographic methods (11,12). Many of these advantages are also potentially applicable to achiral stationary phases; however, achiral SFC lacks a universal stationary phase equivalent to the C18 phase typically leveraged in reversed-phase liquid chromatography. Instead, SFC has borrowed much of its initial stationary phase chemistry from approaches that have been successful in normal-phase liquid chromatography. Newer purpose-built chemistries, such as pyridyl-type stationary phases, have become more prevalent over the past decade (13). With this has come a need to evaluate which set of phases would be most likely to reveal advantageous and complementary selectivity for the analytes of interest. With the current myriad of chemistries available, the task of making this selection has become very daunting (14)
Methods and guidance have been proposed to enable SFC practitioners to decide which stationary phases should be chosen as part of a common column library screening kit (14–18). Orthogonality, or dissimilarity, is one of the quantitative metrics used to study differential selectivity between stationary phases. If a set of columns shows relatively high orthogonality to each other, then they retain analytes differently so as to yield different separation order (or resolution) for the representative sample mixture tested. Ideally, this metric can be used to increase the probability that a separation on at least one column will occur during the course of a screen, while employing a minimal set of stationary phases. Thus, it is best to maximize orthogonality of a screening kit for this purpose (19). Lesselier, West, and associates have published very comprehensive and informative spider diagrams that utilize properties of the linear solvent energy relationship to tease out differences between classes of stationary phases (14–16). Galea and colleagues combine information from r (correlation coefficient)-color maps, principle component analysis, and weighted average linkage clustering to determine classes of stationary phases (20). These were ultimately defined by the following six columns: C18, silica, amino, cyano, phenyl, and HILIC (diol-type). There was a fundamental difference in analyte classes studied between these two sets of authors, as mentioned by Galea and associates; the former used benzenic, naphthenic, and polyaromatic hydrocarbon type compounds, and the latter used pharmaceutical-type compounds. McClain and colleagues also demonstrated the viability of different stationary phases relative to analyte good separation and peak shape for analyte structural classes, such as amides, alcohols, acids, and amines (21). The results of this work led to the development of four column classes: basic (imidazole-based), ethyl pyridine, diethylaminopropyl (DEAP), and nitro. There is some suggestion that common phases like diol, cyano, aromatic-type, and nitrogen-containing phases are sufficiently different enough to warrant consideration in screening. In this regard, consistency in screening selection methods and more application studies are always welcome.
In this work, we use gradient screening conditions to examine the efficacy of using seven different stationary phase chemistries: naphthyl, nitro, pyridyl amide, basic, ethyl pyridine, cyano, and diol. Eighteen pharmaceutically related compounds were chosen to evaluate the gradient retention and selectivity. Further, the overall orthogonality of each stationary phase was tested to determine if there is any similarity relative to the analytes tested. Finally, a mixture of 18 polycyclic aromatic hydrocarbons (22) was used to test separation performance against a single class of analytes and determine overall differences in retention for π-π interactions.
Experimental
Instrumentation
All SFC screening runs were performed on an Agilent Analytical 1100 SFC system (Agilent Technologies, Santa Clara, California). The system included a Fusion A5 SFC control module (400 bar maximum), degasser (G1322A), SFC binary pump (G1312A), SFC ALS auto-sampler (G1313A), thermostatted column compartment (G1316A, up to 80 °C), a 6-column selector (G1159A), and a ultraviolet-visible (UV–vis) diode array detector (DAD) (G1315A, 190–950 nm). The column heater (HOT-2012L) was fitted with a 25 cm thermal jacket (both from Analytical Sales and Services, Inc., Flanders, New Jersey). The SFC system was controlled using OpenLab CDS ChemStation edition software (Rev. C.01.07 SR2).
Chemicals and Reagents:
Analytes used in this study include caffeine, flavanone, and theophylline from Acros Organics (Morris Plains, New Jersey); ketoprofen from Ark Pharm, Inc. (Arlington Heights, Illinois); p-toluamide from Combi-Blocks, Inc. (San Diego, California); ibuprofen from MP Biomedicals (Santa Ana, California); 1,1'-bi-2-naphthol, 1,3,5-tri-tert-butylbenzene, benzoin, coumarin, naphthalene, n-benzylbenzamide, nicotinamide, (S,R)-noscapine, sorbic acid, sulfamethizole, trans-stilbene oxide (TSO), and Tröger's base from Sigma-Aldrich (St. Louis, Missouri). Optima-grade methanol and acetonitrile from Fisher Scientific (Fair Lawn, New Jersey) was used as a solvent. Bone dry-grade CO2 was obtained from Airgas (Randor Township, Pennsylvania). EPA method 8310 polycyclic aromatic hydrocarbon (PAH) mixture was purchased from Restek Corp. (Bellefonte, Pennsylvania).
Screening Parameters
Pure methanol was utilized as a modifier for all screenings. After any column switch, the stationary phase was initially equilibrated for 40 min at 10% modifier prior to use (to ensure reliable retention during the first run). All subsequent runs on the same column only required a 3 min equilibration. Each sample mixture was analyzed in triplicate. A gradient from 10 to 50% modifier was performed for 10 min, followed by a 3 min isocratic hold at 50%, to elute any heavily retained analytes. Flow rate was maintained at 2 mL/min throughout each run. The pre- and post-column mantles were set to 40 °C and 37.5 °C, respectively. The column thermal jacket was heated to 40 °C. The backpressure regulator (BPR) was set to 100 bar and a temperature of 50 °C. Analyte detection was performed solely by DAD. The DAD was set to a wavelength of 210 nm with a bandwidth of 4 nm, a sampling rate of 10 Hz, and a background wavelength of 360 nm with a 100 nm bandwidth. Confirmation of analyte identification was performed by eluting individual analytes.
Sample Preparation
All samples (excluding the PAH reference mixture) were prepared at concentrations of 100 µg per 1 mL of methanol solution for each analyte (including both mixtures and individual standards). These solutions were sonicated for approximately 10 min, and filtered through a 0.45 µm syringe filter prior to use. When not in use, samples were stored at 4 °C, to maintain stability. The PAH reference mixture was received as a solution of 18 PAH analytes, with a concentration of 500 µg/mL each, dissolved in 92:8 (v/v) acetonitrile:toluene. A 1:10 dilution in pure HPLC grade acetonitrile was performed to produce the final sample mixture.
Columns
Seven columns were screened with dimensions of 4.6-mm × 150-mm (internal diameter × length) and 5 µm fully porous particles. The stationary phases were ethyl pyridine, naphthyl, nitro, cyano, diol, basic, and pyridyl amide (ES Industries, Inc., West Berlin, New Jersey). The basic column consists of imidazole-based moieties in its structure, but will be referred to as basic in the rest of the text for consistency. Only the ethylpyridine column is endcapped. The surface area for all sorbents is reported to be 275 m2/g. The stationary phase chemistries are depicted in Figure 1.
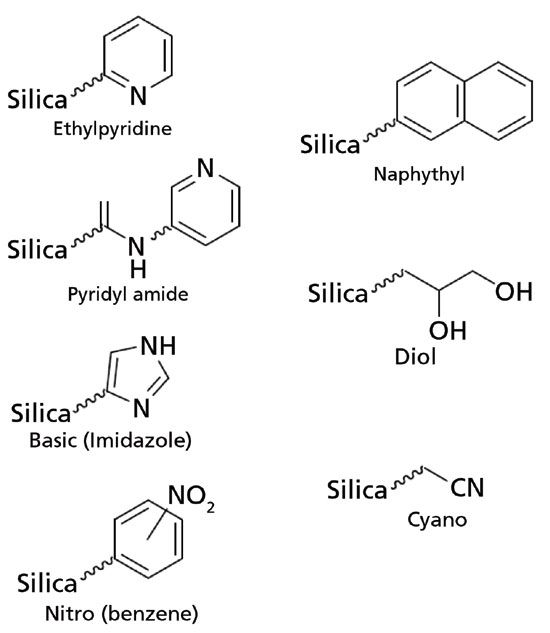
Figure 1: Stationary phase generic names, and their corresponding chemistries used for the achiral SFC study.
Calculations
Values for cLogP and pKa were determined using ACDLabs V2015. All other calculations were performed in Microsoft Excel, or taken from values exported from the Agilent OpenLab software.
Results and Discussion
Comparing Gradient Retention
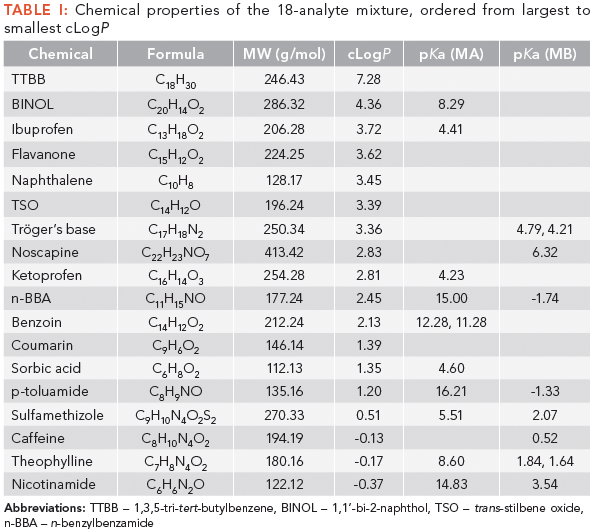
Eighteen analytes were chosen to represent a complex mixture with various degrees of polarity, pKa, and substituted groups (as shown in Figure 2 and Table I). Along with three columns commonly used for stationary phase screening (ethyl pyridine, cyano, and diol) (23–25), four newer stationary phases were also screened, to determine if specific advantage may be afforded. It is worth noting that isocratic separations may yield resolution of a group of analytes that may otherwise completely co-eluted in a gradient, but gradient screening is posited as an initial test for selecting a stationary phase for further method development. As such, choosing a different stationary phase with a consistently simple screening gradient remains preferable to applying a trial-and-error approach to produce an acceptable gradient or isocratic elution.
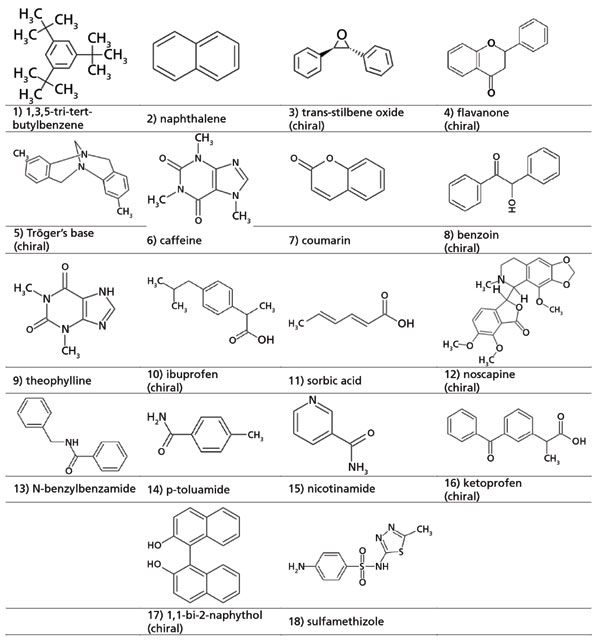
Figure 2: The 18 analytes (both chiral and achiral) used for the screening.
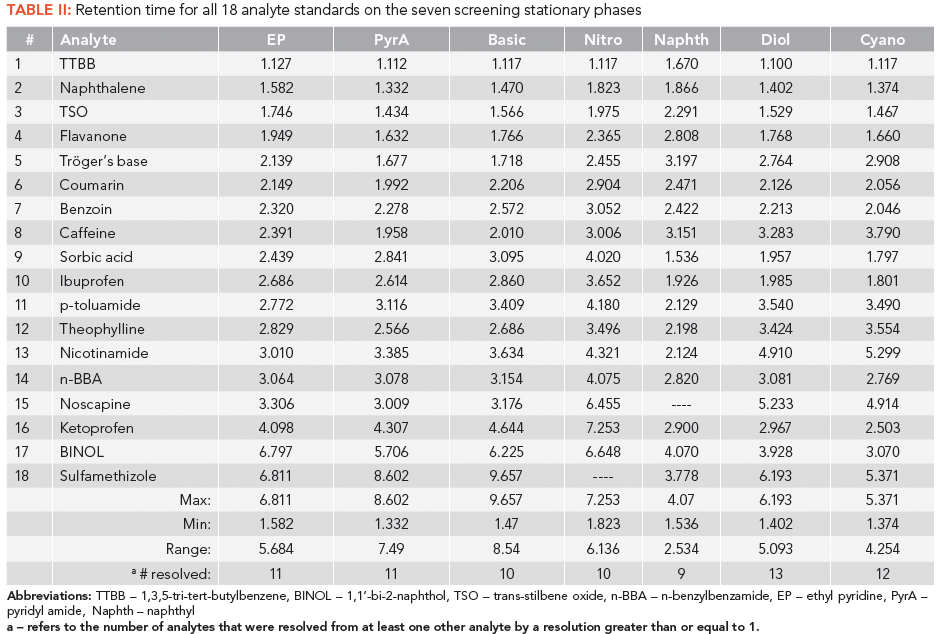
For SFC gradient screening, it is ideal that analytes are first eluted within the retention window of analysis. For the naphthyl and nitro columns, the noscapine and sulfamethizole analytes, respectively, were too highly retained to be detected during the screening window (as shown in Table II). Additionally, noscapine tends to exhibit tailing. The tailing or retentive behavior did not seem to correlate with its cLogP or pKa value (2.83 and 6.32, respectively). Sulfamethizole tended to be the most retained analyte in the mixture for all columns, except for naphthyl. The early-eluted analytes were generally the same between columns as well, including tri-tert-butylbenzene, naphthalene, trans-stilbene oxide, and flavanone (Figure 3). Only the naphthyl stationary phase broke this trend. Curiously, sorbic acid was the first eluted analyte on this column. This is likely because all other 17 analytes have a cyclic ring structure that could be retained on an aromatic-type stationary phase. Speculatively, this property could be useful in separating contaminants or impurities lacking a ring from pharmaceuticals that would engage in strong π-π interactions.
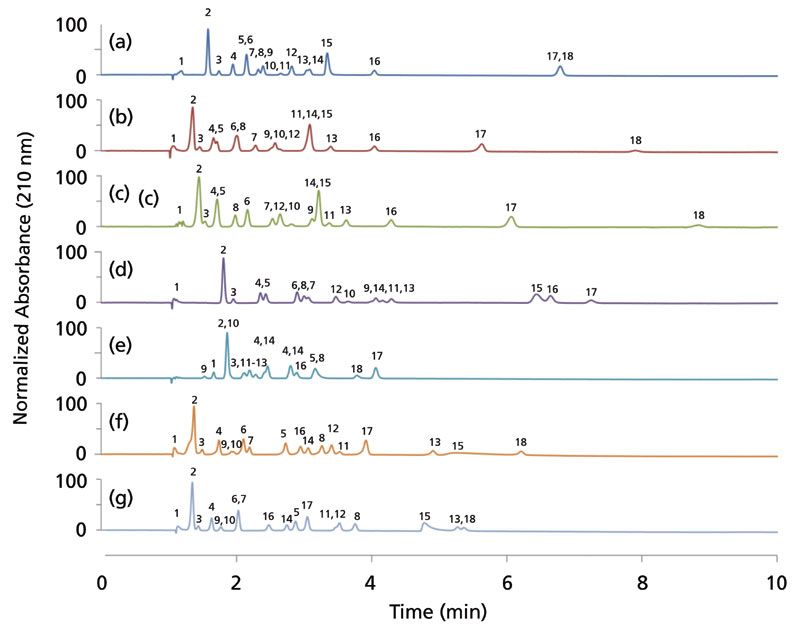
Figure 3: Stacked normalized chromatograms of the 18-analyte mixture of pharmaceutically related compounds using 7 different columns under the same gradient conditions: (a) ethyl pyridine, (b) pyridyl amide, (c) basic (imidazole-based), (d) nitro, (e) naphthyl, (f) diol, and (g) cyano. The column dimensions were 4.6-mm × 150-mm with 5-µm particles. Chromatograms are normalized to the highest intensity analyte peak. Gradient conditions are listed in the "Screening Parameters" section of this article.
Gradient Selectivity and Orthogonality
If the desired components of a sample can be eluted within the gradient screen, and the efficiency of each column in a screen is roughly the same, then selectivity likely becomes the determining factor of a successful separation. Unfortunately, there are more stationary phases available than are feasible for testing in a screening gradient. One way to attenuate the number of stationary phases is to choose those that have selectivity that is most different from each other (or are more "orthogonal"). Orthogonality can be measured from the correlation coefficient (r) of retention factors from each pair of stationary phases. This method was used previously by Galea and associates with the following equation:
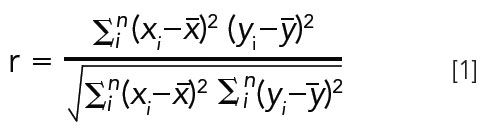
Where n is the number of analytes, i is the analyte index, xi is the ith analyte retention factor on the first (x) stationary phase, yi is the ith analyte retention factor on the second (y) stationary phase, and x with bar and y with bar are the average retention factors of all analytes on the respective stationary phases. As the correlation coefficient approaches one, this indicates a highly similar selectivity for the given analyte set. Values of r closer to zero indicate a high level of orthogonality.
Of the seven stationary phases, the most highly correlated cluster (r ≥ 0.9) is the basic, pyridyl amide, ethyl pyridine, and the nitro (Figure 4). The similarity between the first three stationary phases would be somewhat expected, as they are all bonded with nitrogenous heterocycles (pyridine and imidazole). Cyano and diol also appear fairly similar to one another (r = 0.969), with the highest amount of deviation observed for caffeine and 1,1'-bi-2-naphthol. The naphthyl stationary phase was least similar to all of the other stationary phases. Unlike the other columns, the bonded phase in naphthyl would likely not engage in polar interactions. Rather, any polar interactions would likely only arise from remaining free silanols in the silica support. It is worth mentioning that some of the evaluated stationary phases were designed for the express purpose of not requiring mobile phase additives for improved peak shape (such as GreenSep pyridyl amide and basic), as opposed to focusing on dissimilar selectivity. Developing methods without low volatility additives is preferred for purification purposes if chromatographic performance is considered comparable (26). Thus, orthogonality may not be the only consideration for choosing column screening candidates for purification.
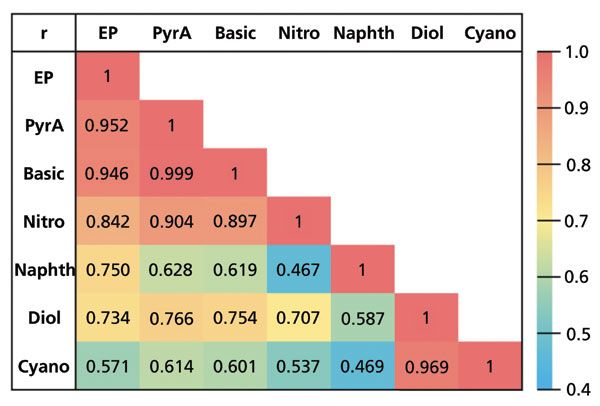
Figure 4: Correlation coefficient (r) matrix for each pair of the seven stationary phases. The highest correlation is represented by r = 1 (red), which is shown along the diagonal. Abbreviations: EP – ethyl pyridine, PyrA – pyridyl amide, Naphth – naphthyl; r is Pearson's correlation coefficient.
PAH Reference Mixture
A reference standard mixture of polycyclic aromatic hydrocarbons was used to represent a sample with a number of analytes equal to the previous test mixture (18 analytes), but all of a specific class. Analytes were not individually identified, but the number of peaks resolved (with Rs ≥ 1) was used as a basis for screening performance on a nonpolar analyte set. A large void volume peak was detected on all chromatograms (Figure 5). This was likely due to the presence of toluene (8 vol%) in the initial reference solvent. The pyridyl amide and basic stationary phases yielded quite similar chromatograms, with one additional peak resolved by Pyridyl amide. The PAHs were poorly separated on the diol and cyano, with only nine and six analytes being resolved on each, respectively. Interestingly, the diol column yielded similar retention to basic and pyridyl amide, but seemed to have slightly poorer selectivity for a couple of critical pairs. The cyano column appeared to suffer from an inadequately small retention window for the PAH mixture. The highest retention and separation performance was obtained by ethyl pyridine, naphthyl, and nitro stationary phases, with each resolving 14 analytes within the screening time. The excellent selectivity offered by naphthyl would be expected for PAHs, given that it is bonded to a PAH. Nitro yielded the highest retention, with two analytes eluting outside of the screening window. Because the effective carbon load and surface area for each column is unknown, the increased retention cannot be solely attributed to higher π-π interactions.
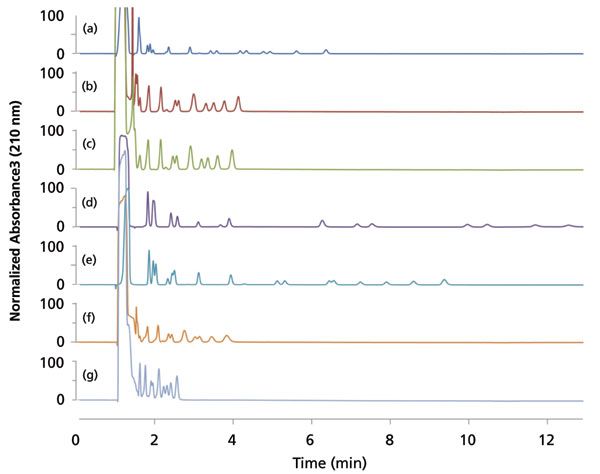
Figure 5: Stacked normalized chromatograms of the 8310 polycyclic aromatic hydrocarbon (PAH) reference standard on 7 different columns under the same gradient conditions: (a) ethyl pyridine, (b) pyridyl amide, (c) basic (imidazole-based), (d) nitro, (e) naphthyl, (f) diol, and (g) cyano. The number of analytes resolved (Rs ≥ 1) for each column was 14, 12, 11, 14, 14, 9, and 6, respectively. The column dimensions were 4.6-mm × 150-mm with 5-µm particles. Chromatograms were normalized to the highest intensity between 1.5 and 13 minutes to eliminate scaling issues due to the high absorbance solvent peak. Gradient conditions are listed in the Screening Parameters section.
Conclusion
Seven stationary phases were evaluated for performance of two separate 18-analyte mixtures. The diol and cyano stationary phases resolved the highest number of analytes. These two stationary phases also appeared to show strong correlation in retention factors. Correlation was also very high for the pyridyl amide, basic, and ethylpyridine stationary phases. Though the naphthyl column displayed the worst performance for these analytes, it was also the least similar to any of the other six stationary phases. For the 18-analyte PAH mixture ethylpyridine, nitro, and naphthyl resolved the highest number of analytes. Of these, the naphthyl column had by far the highest retention of PAH analytes, which may be advantageous if leveraging π-π interactions to obtain selectivity between analytes of interest. Ultimately stationary phases like naphthyl and nitro should be considered if selectivity or retention is not satisfactory with cyano, diol or pyridyl-type column, providing orthogonal separation conditions and expanding the complementarity of achiral SFC screenings.
Acknowledgments
The authors thank the MRL Postdoctoral Research Fellows Program for financial support and the MRL Department of Process Research and Development for making available the use of the instrumentation used in this study.
References
(1) C.L. Barhate, L.A. Joyce, A.A. Makarov, K. Zawatzky, F. Bernardoni, W.A. Schafer, D. W. Armstrong, C.J. Welch, and E.L. Regalado, Chem. Commun. 53(3), 509–512 (2017).
(2) M. Catani, O.H. Ismail, F. Gasparrini, M. Antonelli, L. Pasti, N. Marchetti, S. Felletti, and A. Cavazzini, Analyst 142(4), 555–566 (2017).
(3) C.L. Barhate, M.F. Wahab, D.J. Tognarelli, T.A. Berger, and D.W. Armstrong, Anal. Chem. 88(17), 8664–8672 (2016).
(4) C.L. Barhate, D.A. Lopez, A.A. Makarov, X. Bu, W.J. Morris, A. Lekhal, R. Hartman, D.W. Armstrong, and E.L. Regalado, J. Chromatogr. A 1539, 87–92 (2018).
(5) O.H. Ismail, G.L. Losacco, G. Mazzoccanti, A. Ciogli, C. Villani, M. Catani, L. Pasti, S. Anderson, A. Cavazzini, and F. Gasparrini, Anal. Chem. 90(18), 10828–10836 (2018).
(6) E.L. Regalado, C.J. Welch, Trends Anal. Chem. 67, 74–81 (2015).
(7) A. Dispas, R. Marini, V. Desfontaine, J.-L. Veuthey, D. Kotoni, L.G. Losacco, A. Clarke, C. Muscat Galea, D. Mangelings, B.M. Jocher, E.L. Regalado, K. Plachká, L. Nováková, B. Wuyts, I. François, M. Gray, A.J. Aubin, A. Tarafder, M. Cazes, C. Desvignes, L. Villemet, M. Sarrut, A. Raimbault, E. Lemasson, E. Lesellier, C. West, T. Leek, M. Wong, L. Dai, K. Zhang, A. Grand-Guillaume Perrenoud, C. Brunelli, P. Hennig, S. Bertin, F. Mauge, N. Da Costa, W.P. Farrell, M. Hill, N. Desphande, M. Grangrade, S. Sadaphule, R. Yadav, S. Rane, S. Shringare, M. Iguiniz, S. Heinisch, J. Lefevre, E. Corbel, N. Roques, Y.V. Heyden, D. Guillarme, and P. Hubert, J. Pharm. Biomed. Anal. 161, 414–424 (2018).
(8) V. D'Atri, S. Fekete, A. Clarke, J.-L. Veuthey, and D. Guillarme, Anal. Chem. 91(1), 210–239 (2019).
(9) V. Desfontaine, G.L. Losacco, Y. Gagnebin, J. Pezzatti, W.P. Farrell, V. González-Ruiz, S. Rudaz, J.-L. Veuthey, and D. Guillarme, J. Chromatogr. A 1562, 96–107 (2018).
(10) L.T. Taylor, J. Supercrit. Fluids 47(3), 566–573 (2009).
(11) H. Shaaban and T. Górecki, Talanta 132, 739–752 (2015).
(12) J. Liu, E.L. Regalado, I. Mergelsberg, and C.J. Welch, Org. Biomol. Chem. 11(30), 4925–4929 (2013).
(13) R. McClain, M.H. Hyun, Y. Li, and C.J. Welch, J. Chromatogr. A 1302, 163–173 (2013).
(14) C. West, E. Lemasson, S. Bertin, P. Hennig, and E. Lesellier, J. Chromatogr. A 1440, 212–228 (2016).
(15) C. West and E. Lesellier, J. Chromatogr. A 1191(1), 21–39 (2008).
(16) E. Lesellier and C. West, J. Chromatogr. A 1382, 2–46 (2015).
(17) E.L. Regalado, W. Schafer, R. McClain, and C.J. Welch, J. Chromatogr. A 1314, 266–275 (2013).
(18) E.L. Regalado, P. Zhuang, Y. Chen, A.A. Makarov, W.A. Schafer, N. McGachy, and C.J. Welch, Anal. Chem. 86(1), 805–813 (2014).
(19) K. Ebinger and H.N. Weller, J. Chromatogr. A1332, 73–81 (2014).
(20) C. Galea, D. Mangelings, and Y.V. Heyden, J. Pharm. Biomed. Anal. 111, 333–343 (2015).
(21) R. McClain and M. Przybyciel, LCGC Europe 25(10), 5776-5584 (2012).
(22) P.G. Stevenson, S. Kayillo, G.R. Dennis, and R.A. Shalliker, J Liq. Chromatogr. R.T. 31(3), 324–347 (2007).
(23) C. West, S. Khater, and E. Lesellier, J. Chromatogr. A 1250, 182–195 (2012).
(24) S. David, C. David, and A. Jean-Michel, Curr. Top. Med. Chem. 12(11), 1250–1263 (2012).
(25) C.F. Poole, J. Chromatogr. A1250, 157–171 (2012).
(26) M. Ventura, B. Murphy, and W. Goetzinger, J. Chromatogr. A 1220, 147–155 (2012).
Raffeal Bennett, Erik L. Regalado, and Mirlinda Biba are with the Process Research and Development Department at Merck & Co., Inc., in Rahway, NJ. Direct correspondence to: raffeal.bennett@merck.com or mirlinda_biba@merck.com
Matthew Przybyciel is with ES Industries, in West Berlin, NJ.

Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.

















