Enhancement of UV Detection Sensitivity in SFC Using Reference Wavelength Compensation
Regulatory requirements for the identification, quantification, and control of impurities in drug substances and their formulated products are increasingly being explicitly defined, particularly through the International Conference of Harmonization (ICH).
Regulatory requirements for the identification, quantification, and control of impurities in drug substances and their formulated products are increasingly being explicitly defined, particularly through the International Conference of Harmonization (ICH). According to ICH, the threshold for identification and qualification of organic impurities is 0.1% for the majority of compounds, which implies a limit of quantification (LOQ) of 0.05% will be required for the involved analytical technology. With an increasing number of single enantiomers and stereoisomers being developed as drug candidates, detection and quantitation of chiral impurities to the 0.1% level are of great importance. Supercritical fluid chromatography (SFC) is a superior chromatographic technique for chiral separation; however, traditionally SFC UV has not been considered a highly sensitive technique.
While much effort has been applied to hardware improvement, using appropriate reference wavelength(s) compensation in data acquisition, a common built-in feature of photo diode array (PDA) detectors and the like, offers a facile means to effectively reduce most non-wavelength-dependent noise; and thereby increase the overall signal/noise ratio (S/N). Reference wavelength compensation collects wide-band absorbance data in a region where the analytes have minimal or no absorption. The detector calculates the compensation value by averaging the absorbance values within the selected range of wavelengths. The averaged value is then subtracted from the absorbance value. Since the main absorbance includes the reference bands, noises from common sources, can be effectively reduced. The closer the reference bands are to the λmax of the analyte of interest, the more effective the noise reduction will become.
In this application note, we demonstrate the enhancement of UV detection sensitivity in SFC by using reference wavelength compensation, a built-in feature of Waters 2998 PDA under both Masslynx™ and Empower™.
Experimental
All experiments were carried out using a Thar SFC MS Resolution (TharSFC, a Waters company, Pittsburgh, PA). The system consists of a fluid delivery module (FDM), Alias® autosampler, column oven, automated back pressure regulator (ABPR), Waters 3100 mass detector, and Waters 2998 photodiode detector (PDA). Masslynx™ was used for data acquisition and analysis. In all experiments, the sampling rate for the 2998 PDA was 5 points/s, resolution was 3.6 nm, and the filter constant was set at "slow."

Figure 1
For the experiments of hydrocortisone, caffeine, acetaminophen, and sulfamerazine, a silica column (4.6 × 50 mm) was used. The flow rate was set at 3 mL/min; the system pressure was set at 150 bar; the temperature was 40 °C; and the injection volume was 5 μL (full loop). An isocratic method of 25% methanol was used. The compensated wavelengths were as follows: hydrocortisone (290 to 330 nm); caffeine (310 to 350 nm); acetaminophen (310 to 350 nm); and sulfamerazine (310 to 350 nm).
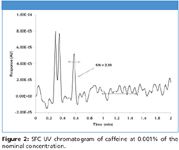
Figure 2
For the warfarin experiments, an OD-H column (4.6 × 250 mm) was used. The flow rate was set at 3 mL/min; the system pressure was set at 150 bar; the temperature was 40 °C; and the injection volume was 5 μL (full loop). An isocratic method of 30% methanol with 0.4% dimethylethanolamine (DMEA) amine was used. The compensated wavelength range was 330 to 370 nm.
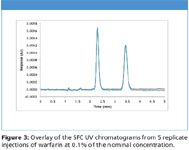
Figure 3
All samples were dissolved in methanol. The concentrations for all compounds were as follows: warfarin (5 mg/mL, or 2.5 mg/mL for each enantiomer); hydrocortisone (2.5 mg/mL); caffeine (2.0 mg/mL); acetaminophen (2.0 mg/mL); and sulfamerazine (2.0 mg/mL).

Figure 4
Results and Discussion
Figure 1 shows a comparison of two hydrocortisone chromatograms at 0.125 μg/mL. Figure 1A is a standard chromatogram at γmax, whereas figure 1B is a chromatogram processed using reference wavelength compensation. While the peaks at 0.6 min have similar heights, the S/N of 1B is almost 4 times higher than 1A, suggesting that reference wavelength compensation provides a 4-fold reduction in noise. On average, a minimum 3~5 fold increase in S/N was obtained in all compounds tested. Another example of reference wavelength compensation is shown in figure 2 with caffeine at a concentration close to its LOD (0.25 μg/mL).
Next we demonstrate the quantitative analysis of warfarin by SFC UV. Figure 3 shows the overlay of SFC UV chromatograms from 5 replicate injections of warfarin at 0.1% of the nominal concentration. Excellent reproducibility was achieved on both retention time and peak area (Table I). At this concentration, the average S/N is above 100. Figure 4 is the SFC UV chromatogram of warfarin at 0.005% of the nominal concentration. This concentration represents 0.000625 μg (0.625 ng) of each enantiomer on the column. Even at this low concentration, the S/N is still above 10 for peak 1, and slightly lower than 10 for peak 2.

Figure 5
Figure 5 shows the calibration curves for both peak 1 and peak 2, with a correlation coefficient of 1 for both curves. The linearity range expands from 0.005% to 100%, over 4 orders of magnitude, of the nominal concentration. The curves for the two enantiomers also displayed excellent agreement between each other.
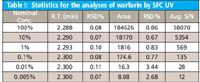
Table I: Statistics for the analyses of warfarin by SFC UV
Conclusion
Using the built-in feature of Waters 2998 PDA, reference wavelength compensation, an average 3~5 fold increase in S/N was achieved for all tested compounds, indicating a 3~5 fold reduction in noise. Using reference wavelength compensation, an LOQ of 0.125 μg/mL (0.625 ng on the column) of each enantiomer of warfarin and over 4 orders of magnitude of linearity were obtained, the best sensitivity and widest dynamic range ever reported in SFC. These results indicate SFC is ready for the prime time and suitable for use in the analysis of impurities, enantiomeric excess (EE) determinations, and QA/QC.
In LCGC's June Application Notebook, "Profiling the Isomerization of Biologically Relevant (E)-(Z) Isomers by Supercritical Fluid Chromatography (SFC)," p. 24, several Thar authors were not listed. The correct author listing should be Jacqueline Cole1 , Vasiliy N. Korotchenko2 , Billy W. Day2, and Rui Chen1 , 1 TharSFC, a Waters Company, 2 Department of Pharmaceutical Sciences, University of Pittsburgh. BWD and VNK would like to thank the NIH (grant number HL088016) for the financial support.

TharSFC, a Waters Company
575 Epsilon Drive, Suite 100, Pittsburgh, PA 15238
tel. (412)967-5665; fax (412)967-9446
Email: info@tharsfc.com
Website: www.waters.com/sfc

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.















