Developing and Validating Dissolution Procedures
LCGC North America
This month's "Validation Viewpoint" installment highlights some method validation guidelines used in developing and validating dissolution test procedures.
The dissolution performance test is a required test for all solid oral dosage forms for product release testing. It also is used commonly as a predictor of a drug product's in-vivo performance. To help satisfy dissolution requirements, the USP provides information in the way of a general chapter on dissolution, as well as related chapters on disintegration and drug release (1–3). The USP and the FDA also provide guidelines on development and validation of dissolution procedures (4–9), and while this month's column will draw from this information and will discuss the available guidance in some detail, we encourage readers to consult the references for additional details.

Michael E. Swartz
In-vitro dissolution data, together with bioavailability, and chemistry, manufacturing, and control (CMC) data, is a critical component of any new drug application (NDA) submitted to the FDA. A dissolution test is really a simple concept; a tablet or capsule is placed into a known volume of media, and as it dissolves the resulting solution is sampled over time, and assayed (often by high performance liquid chromatography [HPLC], but also by spectrophotometry) for the level of active pharmaceutical ingredient (API) present. However, the design, development, and validation of the procedure can be quite involved, especially when one considers that not only must the dissolution procedure be developed and validated, but also any analytical technique used for the assay.

Ira S. Krull
Qualification and Calibration
Before undertaking the task of dissolution procedure development and validation, it is necessary to invest some time and energy up front to ensure that the dissolution system itself is validated, or qualified. Qualification is a subset of the overall validation process that verifies proper module and system performance before the instrument is placed on-line in a regulated environment (10–13). Analysts for years have used prednisone and salicylic acid tablets to qualify and "chemically" calibrate dissolution instruments. Figure 1 illustrates example HPLC methods commonly used for this purpose.

Recent FDA guidelines suggest that alternative mechanical calibrations also can be used and, when properly executed, satisfy cGMP requirements (14,15).

Figure 1
Dissolution Procedure Development
The dissolution procedure has several distinct components. These components include a dissolution medium, an apparatus, the study design (including acceptance criteria), and the mode of assay. All of these components must be properly chosen and developed to provide a method that is reproducible for within-laboratory day-to-day operation and robust enough to enable transfer to another laboratory.
The Dissolution Medium
Selection of the most appropriate media conditions is based upon discriminatory capability, robustness, stability of the analyte in the test medium, and relevance to in-vivo performance, where possible. When selecting the dissolution medium, physical and chemical data for the drug substance and drug product must be considered — for example, the solubility and solution state stability of the drug as a function of the pH value. Other critical drug product properties include the release mechanism (immediate, delayed, or modified) and disintegration rate as affected by formulation hardness, friability, presence of solubility enhancers, and presence of other excipients. When selecting the composition of the medium, the influence of buffers, molarity, pH, and surfactants on the solubility and stability of the drug also should be evaluated.
The most common dissolution medium is probably dilute hydrochloric acid; however, other media used include buffers in the physiologic pH range of 1.2 to 7.5, simulated gastric or intestinal fluid (with or without enzymes), water, and surfactants (with or without acids or buffers) such as polysorbate 80, sodium lauryl sulfate, and bile salts. The use of aqueous–organic solvent mixtures, while generally discouraged, also can be used if justified. Sometimes enzymes also are used in the media when testing gelatin capsule products.
Media volumes are typically in the range of 500–1000 mL, with 900 mL the most common volume. Volumes as high as 2–4 L have been used, and they can be as low as 100 mL for high potency (low dosage strength) drug formulations. Media deaeration usually is required and can be accomplished by heating the medium or (more commonly) filtering the medium or placing it under vacuum for a short period of time. USP chapter 711 contains additional information on deaeration (2). During method development, results from dissolution samples run in a nondeaerated medium versus a deaerated medium should be compared to determine whether deaeration is necessary.
When developing a dissolution procedure, one general goal is to have "sink" conditions. Sink conditions are defined as the volume of medium that is at least three times that required to form a saturated solution of drug substance. Dissolution results will reflect the properties of the dosage form more accurately when sink conditions are present.
The Dissolution Apparatus
USP chapter 711 lists seven types of dissolution apparatuses (2). The choice of apparatus is based upon the dosage form performance in the in-vitro test system. For solid oral dosage forms, the most frequently used apparatus are Apparatus 1 (basket) and Apparatus 2 (paddle). Additional apparatus used include: Apparatus 3 (reciprocating cylinder), which is especially useful for bead-type modified-release dosage forms; Apparatus 4 (flow-through cell), which has advantages for modified-release dosage forms that contain active ingredients with limited solubility (both Apparatus 3 and Apparatus 4 also can have utility for soft gelatin capsules, bead products, suppositories, or poorly soluble drugs); Apparatus 5 (paddle over disk) and Apparatus 6 (rotating cylinder), which have been shown to be useful for evaluating and testing transdermal dosage forms; and Apparatus 7 (reciprocating holder), which has been shown to have application to nondisintegrating oral modified-release dosage forms, as well as to transdermal dosage forms. Somewhat recently, an AAPS committee published recommendations for the type of apparatus recommended for novel or special dosage forms (16). These recommendations are summarized in Table I. While changes to the approved apparatuses are allowed, justification must be provided.
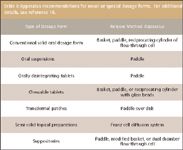
Table I: Apparatus recommendations for novel or special dosage forms. For additional details, see reference 16.
For some dosage forms, particularly capsules that might float on the media surface, "sinkers" might be required. While mentioned in USP chapter 711 (2), USP chapter 1092 provides additional detail for constructing and using sinkers (4). If sinkers are required, steps must be taken in method development to evaluate different types and construction, as sinkers can affect dissolution significantly.
Agitation is also an important part of the dissolution procedure. Apparatus 1 (baskets) at 100 rpm or Apparatus 2 (paddles) at 50 or 75 rpm are used most commonly. Other agitation speeds and apparatuses are acceptable with appropriate justification obtained during method development. Higher or lower rates are usually inappropriate because of the inconsistency of hydrodynamics below 25 rpm and increased turbulence above 150 rpm. Coning or mounding problems can be solved by increasing the paddle speed. If justified, 100 rpm can be used, especially for extended-release products. Decreasing or increasing the apparatus rotation speed also can be justified if the dissolution profiles better reflect in-vivo performance, or if the method results in better discrimination between bulk batch properties without adversely affecting method reproducibility.
Apparatus site selection is also important; vibrations from doors closing or pumps (for example, mass spectrometry [MS] instrument vacuum pumps) can cause significant variability.
Dissolution Study Design
Dissolution is evaluated by measuring rate release profiles, or the amount dissolved over time. Single or multiple points in time can be measured, depending upon the dosage type or data desired. For immediate-release dosage forms, the procedure duration is usually 30 –60 min; and in most cases, a single time point specification is adequate. However, for formulation development comparison purposes, profile comparisons are required, and it is common to collect data from numerous time points, for example, every two minutes or less over the course of the test. For profile comparisons, a sufficient number of time points should be selected to adequately characterize the dissolution curve ascending and plateau.
For an extended-release dosage forms, at least three test time points typically are chosen to characterize the in vitro drug release profile. An early time point, usually 1–2 h, is chosen to show that there is little probability of dose dumping (too much drug product dissolving too soon). An intermediate time point is chosen to define the in-vitro release profile of the dosage form, and a final time point is chosen to show the essentially complete release of the drug. Test times and specifications usually are established on the basis of an evaluation of drug release profile data. For products containing more than a single active ingredient, drug release is to be determined for each active ingredient.
Sampling is another important experimental design consideration. For many tests, particularly immediate release formulation tests using one time point over a short (less than 1 h) period, sampling can be done manually. For extended tests, tests with multiple sampling times, or to increase throughput, automated sampling is a useful alternative. When automated sampling is employed, it is important to determine that no bias versus the manual method has been introduced. Regardless of the method of sampling, the sampling site must conform to specifications in the USP (2). Any hydrodynamic disturbance of the vessels by the sampling probes also should be considered, and adequate validation should be performed to ensure that the probes are not introducing a significant change in the dissolution rate.
Filtration also should be considered during the method development or experimental design. Dissolution sample filtration usually is necessary to prevent undissolved drug particles from entering the analytical sample and further dissolving, skewing the test results. Also, filtration removes insoluble excipients that might otherwise cause high background or turbidity in the assay technique.
Acceptance criteria also must be considered during test development. The acceptance criteria should be representative of multiple batches from the same nominal composition and manufacturing process, include key batches used in pivotal studies, and batches that are representative of the drug product performance in stability studies. Acceptance criteria in the form of "Q-factors," or the percentage of the labeled content, are derived, that specify a certain amount dissolved at a given time. Dissolution tests can have a single Q-factor, or might have multiple Q-factors in, for example, an extended release formulation, and are typically in the range of 75% to 80% dissolved. A Q value in excess of 80% generally is not used, because allowance needs to be made for assay and content uniformity ranges. Figure 2 illustrates some example rate release dissolution profiles for an immediate release and an extended release formulation of the same drug substance as determined by HPLC analyses.

Figure 2
Finally, the dissolution test procedure should be discriminating enough to be capable of distinguishing significant changes in a composition or manufacturing process that might be expected to affect in vivo performance. In general, a properly designed dissolution test should result in reproducible data. Too much result variability can make it difficult to identify trends, true batch differences, or effects of formulation changes. If too much variability is observed, the usual remedies include changing the apparatus type, speed of agitation, or deaeration; consideration and examination of sinker type; and changing the composition of the medium. During routine testing of the product, variability outside the expected range should be investigated from analytical, formulation, and processing perspectives.
Assaying the Results
There are two common ways of analyzing dissolution test samples, spectrophotometric (UV) determinations and HPLC. Spectrophotometric determinations are the most common method of analysis because they are faster, simpler, and require less solvent than HPLC. Typically, the drug substance UV spectrum is observed to choose the optimum wavelength for analysis. Cells with pathlengths ranging from 0.02 to 1 cm are used commonly; the smaller-pathlength cells are used to avoid diluting the sample once acceptable linearity and standard error are demonstrated.
HPLC methods, however, have distinct advantages, particularly when there is significant interference from excipients or between multiple active ingredients in the formulation, when increased sensitivity is required, and when there is a desire to automate the dissolution test procedure. HPLC instruments can be used in a flow injection mode when separations are not necessary, and HPLC also has the advantage of different modes of detection (conductivity, fluorescence, and MS for example) for both sensitivity (molecules lacking chromophores) and selectivity purposes. When developing a dissolution procedure that includes an HPLC assay, the compatibility of the dissolution media with the mobile phase must be considered, especially if large injector volumes (over 100 μL) are needed. Single injections of each vessel time point with standards throughout the run constitute a typical run design. Regardless of the mode of assay utilized, however, the procedure must be validated.
Dissolution Procedure Validation
Validation is performed to make sure the method or procedure accomplishes its intended purpose (5,6). Dissolution testing fits into USP category III, which are analytical procedures for the determination of performance characteristics, as summarized in Table II. Because dissolution is a quantitative test, all of the analytical performance characteristics apply, with the exception of the limit of detection. In addition, for HPLC-based assays, system suitability is always required (17). However, in a dissolution test, in addition to the procedure used to perform and assay the test results, some individual "subprocedures" (for example, filtration and solution stability) also must be validated. And while the various validation performance characteristics listed in USP chapter 1225 are well defined in a general sense, the specifics of how the analytical performance characteristics apply to dissolution testing deserves a little more focus.

Table II: Data elements required for general procedure validation (from USP Chapter 1225). Category I: Analytical procedures for quantitation of major components of bulk drug substances or active ingredients (including preservatives) in finished pharmaceutical products. Category II: Analytical procedures for determination of impurities in bulk drug substances or degradation compounds in finished pharmaceutical products. These procedures include quantitative assays and limit tests. Category III: Analytical procedures for determination of performance characteristics. Category IV: Identification tests. An asterisk indicates the parameter may be required, depending upon the nature of the test. For additional details see reference 5.
Specificity–Placebo Interference
To evaluate specificity in dissolution procedures, it is necessary to demonstrate that the results are not affected by placebo constituents, other active drugs, or degradants in the drug product. A proper placebo should consist of everything in the formulation, except the active ingredient: all the excipients and coatings (inks, sinker, and capsule shell also are included when appropriate), other actives, and so forth. In some instances, placebo interference can be evaluated by weighing samples of a placebo blend and dissolving or dispersing it into the dissolution medium at concentrations that would be encountered normally during testing. The interference generally should not exceed 2%.
For extended-release products, a placebo version of the actual drug product might be more appropriate to use than blends, because this placebo formulation will release the various excipients over time in a manner more closely reflecting the product than will a simple blend of the excipients. In this case, it might be appropriate to evaluate potential interference at multiple sampling points in the release profile.
If the placebo interference exceeds 2%, then method modification, such as choosing another wavelength, baseline subtraction using a longer wavelength, or using HPLC might be necessary to avoid the interference. Absence of interfering peaks in the placebo chromatogram or lack of absorbance by the placebo at the analytical wavelength demonstrates specificity.
Linearity and Range
Linearity and range are established by preparing solutions of the drug, ranging in concentration from below the lowest expected concentration to above the highest concentration during release. Typically, solutions are made from a common stock using serial dilutions. A range should be chosen (through appropriate dilutions as necessary) so as not to exceed the linearity limits of the instrument.
Sometimes organic solvents are necessary in the preparation of standards; however, no more than 5% (v/v) of organic solvent in the final solution should be used. Linearity typically is calculated and reported by least-squares linear regression analysis of the curve generated from a minimum of five points. Typically, a square of the correlation coefficient (r2 ≥ 0.98) demonstrates linearity. In addition, the y-intercept must not be significantly different from zero. ICH recommends that for dissolution testing, linearity should be demonstrated ±20% over the range of the dissolution test. For example, for a controlled release drug product with a multiple Q-factor of 20% after one hour, and 80% after 24 h, the validated range should be 0–100% of label claim (6).
Accuracy and Recovery
Accuracy and recovery can be established by preparing samples containing the drug and any other constituents present in the dosage form (for example, excipients, coating materials, and capsule shell) ranging in concentration from below the lowest expected concentration to above the highest concentration during release. ICH recommends a minimum of nine determinations over a minimum of three concentrations — for example, three concentrations, three replicates each. An amount of stock solution equivalent to the targeted label claim can be added to the vessel instead of the drug substance, particularly for very low strengths, as it might be more appropriate to prepare a stock solution than to attempt to weigh very small amounts. The measured recovery is typically 95–105% of the amount added. Often, accuracy and recovery experiments are carried out at the same time as linearity, using data from the same samples.
Precision
For dissolution method validation purposes, precision is measured over two levels, repeatability and intermediate precision. Repeatability refers to the application of the procedure within one laboratory over a short period of time by one analyst using one instrument. Repeatability is determined by replicate measurements of standard and sample solutions. It can be measured by calculating the RSD of the multiple HPLC injections (peak area and retention time) or spectrophotometric readings for each standard solution. Repeatability also can be measured from the same samples used in the accuracy, recovery, and linearity experiments.
Intermediate precision is evaluated to determine the effects of random events on the precision of the analytical procedure. This evaluation typically is done later in the development of the drug product. The use of an experimental matrix design is encouraged to study the effects of different days, analysts, and equipment on precision.
The dissolution profiles on the same sample can be run by at least two analysts, each analyst preparing the standard solutions and the medium. Typically, the analysts use different dissolution baths, spectrophotometers or HPLC equipment (including columns), and autosamplers; and they perform the test on different days.
Acceptance criteria often are calculated from the difference in the mean value between the dissolution results at any two conditions, and specified to not exceed an absolute 10% at time points with less than 85% dissolved and to not exceed 5% for time points above 85%. Acceptance criteria can be product-specific, and other statistical tests and limits can be used.
Robustness
The robustness of an analytical procedure is the measure of its capacity to remain unaffected by small but deliberate variations in parameters internal to the procedure (5,6,18). For dissolution testing, parameters to be varied include medium composition (for example, buffer or surfactant concentration), pH, volume, agitation rate, and temperature. These parameters would be investigated in addition to those typically evaluated during validation of the assay method, either spectrophotometric or HPLC, as discussed in the following section.
Remaining Validation Tests
In addition to the common analytical performance characteristics normally evaluated for procedure validation, standard and sample solution stability and filter validation also must be evaluated. Solution stability is important given the conditions and length of time of some dissolution tests. The standard and sample solution should be stored under conditions that ensure stability. Solution stability is analyzed over a specified period of time, using freshly prepared solutions at each time interval for comparison. The acceptable range for solution stability is typically between 98% and 102%. If the solution is not stable, refrigeration and protection against photodegradation might be needed before sample analysis. A time period for analysis also should be specified. Filter validation is accomplished by preparing a suitable standard solution or a completely dissolved sample solution at the appropriate concentrations. For standard and sample solutions, the results for filtered solutions (after discarding the appropriate volume) to those for the unfiltered solutions can be compared.
Conclusion
Developing and validating dissolution test procedures can be a challenging process, on multiple fronts. Methods must be developed and validated not just for the dissolution test procedure itself, but also for any assay used to evaluate the test results. However, like any task, a systematic and methodical approach taking into account all the components that make up the dissolution test procedure, including the dissolution medium, the choice of apparatus, the test design (including the acceptance criteria), and determining the assay mode will pay great dividends in the end.
Michael E. Swartz "Validation Viewpoint" Co-Editor Michael E. Swartz is Research Director at Synomics Pharmaceutical Services, Wareham, Massachusetts, and a member of LCGC's editorial advisory board.
Ira S. Krull "Validation Viewpoint" Co-Editor Ira S. Krull is an Associate Professor of chemistry at Northeastern University, Boston, Massachusetts, and a member of LCGC's editorial advisory board.
The columnists regret that time constraints prevent them from responding to individual reader queries. However, readers are welcome to submit specific questions and problems, which the columnists may address in future columns. Direct correspondence about this column to "Validation Viewpoint," LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail lcgcedit@lcgcmag.com
References
(1) USP 30-NF 25, December 2007, Chapter 701.
(2) USP 30-NF 25, December 2007, Chapter 711.
(3) USP 30-NF 25, December 2007, Chapter 724.
(4) USP 30-NF 25, December 2007, Chapter 1092.
(5) USP 30-NF 25, December 2007, Chapter 1225.
(6) International Conference on Harmonization, Harmonized Tripartite Guideline, Validation of Analytical Procedures, Text and Methodology, Q2(R1), November 2005, See www.ICH.org.
(7) Guidance for Industry: Dissolution Testing of Immediate Release Solid Oral Dosage Forms, FDA, August 1997. See: www.fda.gov/cder/guidance/1713bp1.pdf
(8) Guidance for Industry: SUPAC-MR: Modified Release Solid Oral Dosage Forms, FDA, September 1997. See: www.fda.gov/cder/guidance/1214fnl.pdf
(9) Guidance for Industry: Waiver of In-Vivo Bioavailability and Bioequivalence Studies for Immediate Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System, FDA, August 2000. See: www.fda.gov/cder/guidance/3618fnl.pdf
(10) AAPS PharmSciTech 2004, 5(1) Article 22 (www.pharmscitech.org)
(11) Pharmacopeial Forum, USP Chapter 1058 (In-process revision), Vol. 31(1) January 2005.
(12) Pharmacopeial Forum, USP Chapter 1058 (In-process revision), Vol. 31(5) August 2005.
(13) M.E. Swartz, Analytical Instrument Qualification, Pharmaceutical Regulatory Guidance Book (Advanstar, Cleveland, 2006), pp. 12-16.
(14) Guidance for Industry: The Use of Mechanical Calibration of Dissolution Apparatus 1 and 2 – Current Good Manufacturing Practice (CGMP), FDA Draft Guidance, October 2007. See: www.fda.gov/cder/guidance/7232dft.htm
(15) FDA Division of Pharmaceutical Analysis, Mechanical Qualification of Dissolution apparatus 1 and 2, Document # DPA-LOP.002, Version 2.0, June 2006. See: www.fda.gov/cder/offices/otr/dissolution.pdf
(16) AAPS PharmSciTech 2003; 4(1) article 7 (see: www.pharmscitech.org).
(17) USP 30-NF 25, December 2007, Chapter 621.
(18) M.E. Swartz and I.S. Krull, LCGC 24(5), 480–490 (2006).

Separating Impurities from Oligonucleotides Using Supercritical Fluid Chromatography
February 21st 2025Supercritical fluid chromatography (SFC) has been optimized for the analysis of 5-, 10-, 15-, and 18-mer oligonucleotides (ONs) and evaluated for its effectiveness in separating impurities from ONs.











