Ion Pairing - Blessing or Curse?
LCGC North America
Reversed-phase liquid chromatography (LC) is a simple and easy-to-use technique that will give satisfactory separations for the samples that most of us encounter. Here, John Dolan examines the pros and cons of this technique.
Reversed-phase liquid chromatography (LC) is a simple and easy-to-use technique that will give satisfactory separations for the samples that most of us encounter. Based upon column sales, somewhere around 70–80% of samples can be separated by reversed-phase techniques. The high-purity silica-based columns available today are quite robust in the 2 < pH < 8 range and there are several choices for columns that will work well up to pH 10. The first choice for most workers is to work at low pH, in the pH 2–3 range. Under these conditions, most acidic samples will have suppressed ionization and be well retained, as will be neutral molecules. Basic analytes will be ionized, but if the bulk of the sample molecule is nonpolar, adequate retention often is obtained. At pH < 10, many basic compounds will not be ionized, so they can be chromatographed successfully, but other bases will still be ionized, so retention can be poor.

John W. Dolan
Columns that contain a polar embedded group, sometimes called "AQ" columns, have come on the market in the last 15 years. These columns allow the use of 100% aqueous mobile phases in the reversed-phase mode. These conditions will sometimes give enough retention of polar basic molecules that would be retained poorly at 5% organic, the lower limit for most reversed-phase columns. In other cases, basic compounds still don't have enough retention, even in 100% aqueous mobile phases or at any pH within the column's pH-stable range. In cases such as this, and when the sample contains a mixture of acids and bases, ion pairing might be a viable option. Ion pairing has plenty of potential problems that discourage its use. These, plus the availability of some specialty "mixed-mode" alternative columns, have made ion pairing much less popular today than it was in the past. However, ion pairing can work magic for some samples. This month's "LC Troubleshooting" will review ion-pair separations.

What is Ion Pairing?
We all know that reversed-phase separation is based upon the nonpolar, or hydrophobic, interaction between nonpolar sample molecules and the nonpolar stationary phase. If the sample molecule has sufficient hydrophobic nature, it will be retained. Retention is adjusted by altering the aqueous-to-organic content of the mobile phase. For example, 30% buffer–70% acetonitrile is less polar, and therefore will reduce retention when compared with 40% buffer–60% acetonitrile. Most samples that are nonpolar (for example, neutral molecules) or can be made nonpolar by adjustment of the mobile phase pH (such as acids at low pH) can be retained sufficiently to allow the development of a satisfactory separation.
When the sample contains ionic components, they can be too polar to be retained by the reversed-phase mode. In such cases, ion pairing can prove to be useful. For example, at low pH, basic samples might be sufficiently polar to be unretained by reversed-phase mode. Enter the ion-pair reagent. An ion-pair reagent is similar to a soap; in fact, ion pairing was called "soap chromatography" by some in its early days. The ion-pair reagent has an ionic end and a nonpolar tail (see Figure 1c), such as in hexane sulfonic acid. The reagent is added to the mobile phase and allowed to come to equilibrium with the column. The nonpolar end of the reagent is held strongly by the nonpolar stationary phase (for example, C8 or C18), leaving the charged functional group sticking out into the mobile phase (Figure 1d). Now ionic species of the opposite charge can be attracted to the immobilized ion-pair reagent, providing chromatographic retention (Figure 1f). (Some people also describe the ion-pair process taking place in the mobile phase, resulting in a neutral ion pairing between the sample molecule and the reagent, which is then retained as a neutral species in the standard reversed-phase manner.)
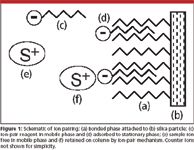
Figure 1
So a negatively charged reagent such as one of the alkyl sulfonic acids can be used to retain positively charged ionic bases. Similarly, a positively charged reagent, such as tetrabutyl ammonium chloride, can be used to retain negatively charged ionic acids. However, there is little incentive to use ion pairing for acids — a simpler and less troublesome solution is available: most acidic compounds can be retained satisfactorily in a low-pH mobile phase.
Developing the Ion-Pair Separation
As is discussed in the following section, if we can avoid using ion pairing, we'll generally have more robust methods, so our first efforts at method development should be to develop a non-ion-pair method. We would proceed as described in the recent method development series by adjusting k-values (retention factors) to the 2–10 range (1). One common indicator that ion pairing might be useful is if, at low pH, some of the compounds behave normally, but others just sit at the column dead time. It is likely that these unretained compounds are ionized bases. If this is the case, ion pairing might be beneficial. Because the addition of an ion-pair reagent to the mobile phase can be thought of as using up some of the reversed-phase character of the column, retention times for neutral compounds will drop when the ion-pair reagent is added. For this reason, it is best to adjust initial k-values so that the first retained peaks have k ≥ 5. This way, the loss of retention for these compounds with the addition of ion-pair reagent will still give k > 1–2 for the final method.
Next, pick an ion-pair reagent. In the present example, we'll assume we have bases present, so we need an acidic ion-pair reagent. Hexane- and octane sulfonate are popular ion-pair reagents for bases. Because these compounds are not highly soluble in acetonitrile, methanol usually is chosen for the organic solvent with ion pairing. There are several ways to approach ion-pair method development — the one I like best is the blending of two solvents, one with and one without ion-pair reagent. Let's assume that the best conditions for the nonbasic compounds is a mobile phase of 60% pH 2.5 phosphate buffer plus 40% methanol. Place this solvent in the A-reservoir. Make up another batch of the same solvent, but add 100 mM hexane sulfonate to it and place it in the B-reservoir. Now, in a stepwise fashion, blend the A- and B-solvents to make mobile phase with increasing ion-pair reagent strengths. For example, start with 0 mM ion-pair reagent, then 10 mM (90% A plus 10% B), 20 mM, and so forth. Allow each mobile phase to equilibrate fully (25–50 mL) before injecting the sample. Inject each sample twice to ensure the retention time is constant. Continue increasing the ion-pair concentration until the retention of the first peak is k > 1. Once the retention times are reasonable, you can fine-tune the peak spacing by adjusting the percent methanol, pH, ion-pair concentration, or temperature in the normal manner (2).
Ion-Pair Precautions
Some of the reputation of ion pairing being problematic is well deserved. The concentration of ion-pair reagent in the stationary phase is controlled by several variables including the organic content of the mobile phase and the column temperature. This means that temperature must be controlled carefully and that gradient elution is difficult, if not impossible. Further compounding the process is the slow equilibration between the mobile phase and the column. Whereas we think 10 column volumes of mobile phase (15–25 mL) is adequate to equilibrate the typical reversed-phase column with a traditional buffer-organic mobile phase, ion-pair reagents can take 20–50 column volumes (50–100 mL) or more to equilibrate. This further discourages gradient elution.
Many ion-pair reagents have substantial UV absorbance, which precludes use with low-UV detection. Also, when the baseline is autozeroed at the beginning of a run, it appears that the baseline is low, but it might be elevated significantly in reality. Whenever anything with lower UV absorbance than the mobile phase enters the column (such as the injection solvent or other reagents in the sample), a negative peak will occur. This means that negative peaks and ghost peaks are common with ion pairing.
Ion-pair reagents can never be washed fully from the column, even with extensive column flushing. This means that you should dedicate a particular column to ion-pair applications. It is alright to switch from one ion-pair reagent to another in a given column, but never use a column for other applications once it has been used for ion pairing. Trace levels of the ion-pair reagent can change selectivity when used for non-ion-pair applications, making column-to-column reproducibility a problem.
Alternatives to Ion Pairing
One alternative to the traditional sulfonate-type ion-pair reagents is to use trifluoroacetic acid. Trifluoroacetic acid is used widely for ion pairing with biological molecules, such as proteins and peptides. It equilibrates quickly and can be used with gradient elution. A standard recipe is to put 0.1% trifluoroacetic acid in water in the A-reservoir and 0.1% trifluoroacetic acid in acetonitrile in the B-reservoir. Now isocratic or gradient methods can be developed in the normal manner, and at low wavelength, if desired.
The AQ or embedded polar phase columns were mentioned earlier. Sometimes these columns under 100% aqueous conditions (with buffer or pH adjustment) will give sufficient retention for polar compounds without the need for ion pairing.
If your sample contains only ionic compounds, you might be able to use ion exchange instead of ion pairing or reversed-phase mode. However, if you have a mixed sample of ionic and nonionic compounds, ion exchange will not be useful.
There are some new columns on the market that contain a mixed-mode stationary phase. One of these (Primesep from SEILC Technologies, Prospect Heights, Illinois) appears similar to a C18 column, but has a positive or negative charge near the base of the C18 chain. This gives the column both ionic and reversed-phase character, as with ion pairing, but does this with reversed-phase reagents (no ion-pair reagent present), so many of the problems of ion pairing can be avoided. Some of these columns allow the user to vary the charge on the column by changing the mobile phase pH.
Conclusions
Ion pairing has justly earned a bad reputation over the years. However, when ion pairing solves your separation problem, many of its shortcomings can be overlooked. Be careful when using ion pairing to allow sufficient time for the mobile phase to equilibrate with the column and avoid changes in temperature or mobile phase organic that can disrupt the equilibrium. Before you embark on developing an ion pairing method, consider carefully if there are alternatives, such as the mixed-mode, AQ, embedded polar phase, or other specialty columns. This discussion has just given a brief overview of ion pairing — for additional information, I recommend reading the ion-pair chapter in reference 3.
John W. Dolan "LC Troubleshooting" Editor John W. Dolan is Vice-President of LC Resources, Walnut Creek, California; and a member of LCGC's editorial advisory board. Direct correspondence about this column to "LC Troubleshooting," LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail John.Dolan@LCResources.com
For an ongoing discussion of LC troubleshooting with John Dolan and other chromatographers, visit the Chromatography Forum discussion group at http://www.chromforum.com.
References
(1) J.W. Dolan, LCGC 25(8), 704-709 (2007).
(2) J.W. Dolan, LCGC 25(9), 944-950 (2007).
(3) L.R. Snyder, J.L. Glajch, and J.J. Kirkland, Practical HPLC Method Development, 2nd ed. (Wiley, New York, 1997).

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.









