Quantifying an Anthrax LF Inhibitor in Human Plasma by HPLC-MS-MS
LCGC North America
This article describes the method development and performance characteristics of the validated LFI assay and evaluates stability in human plasma.
Bacillus anthracis, the etiological agent of anthrax, is a Gram-positive, rod-shaped bacterium that forms spores that are highly resistant to heat, ultraviolet light, radiation, pressure, and chemical agents. The durability of these spores make this bacterium a potential bioweapon (1).
Immediate treatment of anthrax infection with antibiotics is very effective. There is a limited period of time in which an infected patient, who might only exhibit mild flu-like symptoms, can be saved with antibiotic therapy. Toxins released by anthrax cause a patient to succumb to infection well after antibiotics have killed the anthrax bacteria. The major virulence factor of anthrax infection is Lethal Factor (LF), a zinc-dependent metalloprotease toxin. LF disrupts MAP kinase signaling pathways, resulting in cytotoxicity. MAP kinase signaling pathways regulate proinflammatory cytokines. Overproduction of these cytokines is associated with septic shock, respiratory distress, and multiorgan failure leading to death (1–6). Injection of the lethal toxin alone into mammals results in death. Research has demonstrated that inhibiting the activity of LF can reduce tissue damage associated with anthrax infection (7).

LFI ((2R)-2-{[(4-fluoro-3-methyl-phenyl)sulfonyl]amino}-N-hydroxy-2-(tetrahydro-2H-pyran-4-yl)acetamide; Figure 1) previously was shown to be a specific anthrax LF inhibitor (7). A high performance liquid chromatography tandem mass spectrometry (HPLC–MS-MS) method for the determination of LFI in human plasma was developed and validated. HPLC–MS-MS was evaluated as the analytical method. HPLC–MS-MS has become the gold standard for highly selective biological quantitative assays (8,9). LFI and internal standard (ISTD) were extracted from 100 μL of plasma using an automated 96-well liquid–liquid extraction. A C8 HPLC column with a mobile phase consisting of 0.1% acetic acid in water-acetonitrile (80:20, v/v) exhibited superior peak symmetry and retention characteristics for both the LFI and ISTD. The highly aqueous mobile phase was necessary for adequate retention. Both LFI and ISTD were detected using MS-MS with electrospray ionization in the positive mode. The calibration range was 5–1500 ng/mL when 100 μL of plasma was processed. This article describes the method development and performance characteristics of the validated LFI assay and evaluates stability in human plasma.

Figure 1
Experimental
Materials: LFI and the isotopically labeled ISTD (Figure 1) were provided by the Medicinal Chemistry Department (Merck Research Laboratories, West Point, Pennsylvania) and the Labeled Compound Synthesis group in the Drug Metabolism and Pharmacokinetics Department (Merck Research Laboratories, Rahway, New Jersey), respectively. All solvents and reagents were of HPLC or analytical reagent grade and were purchased from Fisher Scientific (Fair Lawn, New Jersey). The drug-free heparinized human plasma was obtained from Biological Specialties (Colmar, Pennsylvania).
Instrumentation: The HPLC–MS-MS system consisted of an Applied Biosystems/MDS Sciex (Foster City, California) API 4000 tandem mass spectrometer equipped with an electrospray ionization interface, a Perkin-Elmer (Norwalk, Connecticut) Series 200 quaternary pump, and a Varian (Palo Alto, California) Pro Star Model 430 autosampler. Data were processed on a Dell Pentium 4 computer using Analyst 1.4 software (Sciex). A Tomtec Quadra 96 (Hamden, Connecticut) liquid-handling robot was used for sample preparation.
HPLC–MS-MS conditions: HPLC separation was performed on a 50 mm ×2.1 mm, 3.5-μm dp Symmetry C8 column (Waters, Milford, Massachusetts) at ambient temperature. The mobile phase was a mixture of 0.1% acetic acid in water–acetonitrile (80:20, v/v) and was delivered at a flow rate of 0.300 mL/min. The retention time was 2.25 min.
The HPLC system was interfaced via electrospray ionization in the positive mode to a PE Sciex API 4000 triple-quadrupole mass spectrometer. The electrospray ionization probe was maintained at 600 °C. Nitrogen gas was used as the nebulizing, auxiliary gas, and collision gas (collision energy of 19 eV). The declustering voltages were 60 V for LFI and 45 V for ISTD. The following transitions were monitored with a dwell time of 400 ms, m/z 347.1→286.1 (LFI) and m/z 351.2→290.2 (ISTD) in Q1→Q3 with collision-induced fragmentation in Q2. Peak-area ratios obtained from multiple reaction monitoring (MRM) of the LFI to ISTD were utilized for the construction of calibration curves, using weighted (1/x2) linear least square regression of the plasma concentrations and the measured peak area ratios.
Standard solutions: LFI working standard solutions, having nominal concentrations of 25, 50, 100, 250, 500, 1000, 2500, 6500, and 7500 ng/mL, were prepared in 1:1 (v/v) water–acetonitrile. An ISTD working solution of 500 ng/mL was prepared by dilution in 1:1 (v/v) water–acetonitrile and was used for all analyses. LFI quality control (QC) samples in control human plasma at nominal concentrations of 15, 300, and 1200 ng/mL also were prepared and stored at -20 °C.

Figure 2
Sample preparation: The final liquid–liquid extraction method consisted of buffering 0.100 mL of plasma with pH 5 acetate buffer, followed by extraction with ethyl acetate using a 96-well plate. The organic layer was transferred to a collection plate and evaporated under a stream of nitrogen at 40 °C. All liquid transfer steps were performed by the Tomtec Quadra 96 workstation liquid handling system. The dried residue was reconstituted with 1.2 mL of mobile phase, and 5-μL aliquots were injected into the HPLC system.

Figure 3
Results and discussion
Q1 and product ion mass spectra: Positive precursor ions [M+H]+ were detected at m/z of 347.1 for LFI and 351.2 for ISTD and the corresponding predominant product ions were detected at 286.1 and 290.2 (Figure 2). Multiple reaction monitoring of these precursor→product ion transitions permitted sensitive and selective detection of the analyte and ISTD.

Table I: Capacity factors of LFI on various HPLC columns with a mobile phase of 0.1% acetic acid in water:acetonitrile (80:20; v/v)
Selection of MS interface and chromatographic conditions: During MS optimization, electrospray ionization in the positive and negative mode and atmospheric pressure chemical ionization in the negative mode exhibited detectable ionization and fragmentation. Monitoring positive ions using electrospray ionization exhibited superior sensitivity (Figure 3) and no significant relative matrix effect (Table V and VI). The use of a stable-labeled internal standard should compensate for ionization efficiency issues in the mass spectrometer source, minimizing potential matrix effect issues (10).

Table II: Assessment of solid-phase extraction of LFI using different sorbents
Chromatographic separation was tested on a variety of 50-mm columns. Different chromatographic conditions were evaluated on several vendors' bonded phases. These included C18, C8, polar embedded, cyano, phenyl, fluorinated, ion exchange, hypercarb, and HILIC sorbents (Table I). Due to the highly polar nature of this analyte, adequate chromatographic retention and good peak shape in the reversed phase was difficult to obtain. Various polar embedded stationary phases were assessed with poor retention. A polar-embedded column with a carbamate linkage exhibited more promising retention. However, it also exhibited poor lot-to-lot reproducibility. The Hypercarb column was too retentive, and reasonable run times could not be obtained with typical solvents. Retention and peak shape were poor on alternative hydrophilic interaction chromatographic columns regardless of mobile phase composition. The Waters Symmetry C8 column provided superior peak shape symmetry and retention characteristics for both LFI and ISTD. A highly aqueous mobile phase was necessary for adequate retention. The capacity factor for both compounds was greater than 2.
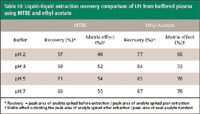
Table III: Liquidâliquid extraction recovery comparison of LFI from buffered plasma using MTBE and ethyl acetate
Sample preparation: In the course of sample preparation development, solid-phase extraction using several alternative sorbents and liquid–liquid extraction using methyl-tert-butyl-ether (MTBE), methylene chloride, and ethyl acetate were assessed. A 96-well liquid–liquid extraction with ethyl acetate provided clean extracts with good recovery. As expected based upon HPLC retention characterisitics, recovery results on traditional solid phase extraction sorbents were poor (Table II). Assessments of liquid–liquid extraction using MTBE and other organic solvents demonstrated low recovery under acidic and neutral conditions (Table III). Ethyl acetate with a polarity index of 4.4, reliably extracted the LFI and ISTD from plasma matrix (Table III). Solvents with low polarity indices minimize the transfer of potential interferences from the aqueous layer. However, this method needed a more polar solvent for adequate, reliable recovery (11,12). The automated 96-well liquid–liquid extraction technique provided high sample throughput at minimal cost.
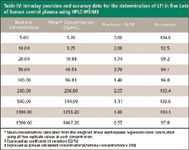
Table IV: Intraday precision and accuracy data for the determination of LFI in five Lots of human control plasma using HPLC-MS/MS
Validation: Intraday validation was performed by replicate analyses of different lots (n = 5) of control human plasma fortified with LFI at the concentrations used to construct calibration curves (5–1500 ng/mL). The chromatographic peak area ratios of product ions for drug and ISTD (drug:ISTD) were obtained, weighted by a factor of 1/x2, and plotted versus the nominal plasma drug concentrations. Table IV presents the validation statistics, namely, precision and accuracy. The data indicate the assay performs well across the entire concentration range. No endogenous interferences were detected in six human plasma lots, establishing assay selectivity. Using the experimental conditions described in this article, the assay LOQ (5 ng/mL) corresponded to approximately 2.1 pg of LFI injected on-column. Representative chromatograms for spiked human control plasma are shown in Figure 4.

Figure 4
Recovery and matrix effect: Extraction recovery and the effect of the sample matrix on ionization efficiency were evaluated in five lots of human plasma. Multiple lots probed for lot-to-lot variability in extraction recovery and matrix ionization efficiency.

Table V: Extraction recovery and assessment of matrix effects on ionization during the determination of LFI and ISTD in human control plasma (n = 5).
Recovery was determined by comparing the absolute peak areas of standards spiked into five lots of human control plasma and extracted by liquid–liquid extraction to the same five lots of control blank plasma extracted under the same conditions and then spiked post extraction with LFI and ISTD ("postextraction samples"). This approach minimizes effects the matrix might have on ionization efficiencies, and reflects only the efficiency of the liquid–liquid extraction process (Table V). Matrix ionization effects are not significant as differences in peak area response between post-extraction samples and neat standards is minimal.
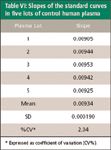
Table VI: Slopes of the standard curves in five lots of control human plasma
The presence of any matrix effect can have an adverse impact on reliable analyte quantification (10,13–15). Matrix enhancement–suppression of ionization was evaluated by comparing the absolute peak areas of postextraction samples to those of neat standards at the same concentrations. Addition of LFI and ISTD to the plasma after the extraction process reflected changes in analyte peak areas due solely to matrix effects on ionization efficiency, and eliminated any variability due to the extraction process itself (Table V). The interlot variability of the absolute peak areas is small (low % CV) for LFI and ISTD, indicating very little relative (interlot) matrix effect. The lack of a relative matrix effect is further illustrated by examination of the slopes of the standard curves in five plasma lots, which resulted in a precision of 2.04 (Table VI).

Table VII: Intraday analysis of plasma quality control samples (QC) spiked LFI in human plasma
Quality Control and Plasma Stability Assessment
Stability of quality control samples was evaluated to ensure the integrity of human clinical samples during bioanalytical analysis. Bulk plasma samples spiked with LFI demonstrated adequate accuracy and precision (Table VII). Stability of LFI in human plasma was established for three freeze-thaw cycles consisting of a thaw to ambient temperature and subsequent refreezing and for storage at ambient temperature for 20 h before processing (Tables VIII and IX).
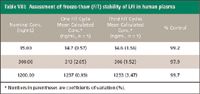
Table VIII: Assessment of freeze-thaw (F/T) stability of LFI in human plasma
Conclusions
An HPLC–MS-MS method for the determination of LFI in human plasma was developed and validated. The absence of relative matrix effects and interferences from the plasma matrix was demonstrated. The highly efficient sample preparation procedure permitted a high-throughput analysis of clinical samples required to support clinical studies. Highly polar compounds can pose difficulties in method development. The use of ethyl acetate in liquid–liquid extraction can provide an effective way to isolate these drugs from aqueous matrices.
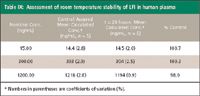
Table IX: Assessment of room temperature stability of LFI in human plasma
Melanie Anderson, Richard Simpson, Laura Archer, and Rajesh Desai, Department of Drug Metabolism, WP75B-300, Merck Research Laboratories, West Point, PA.
Please direct correspondence to Melanie Anderson at melanie_anderson@merck.com
References
(1) A. M. Friedlander, Curr. Clin Top. Infect. Dis. 20, 335–349 (2000).
(2) J.G. Bartlett, T.V. Inglesby, Jr., and L. Borio, Clin. Infect. Dis. 35, 851–858 (2002).
(3) T.V. Inglesby, T. O'Toole, D.A. Henderson, J.G. Bartlett, M.S. Ascher, E. Eitzen, A.M. Friedlander, J. Gerberding, J. Hauer, and J. Hughes, J. Am. Med. Assoc. 287, 2236–2252 (2002).
(4) A. Meyerhoff and D. Murphy, J. Am. Med. Assoc. 288, 1848–1849 (2002).
(5) L.M. Wein, D.L. Craft, and E.H. Kaplan, Proc. Nat. Acad. Sci. USA 100, 4346–4351 (2003).
(6) R. Brookmeyer and N. Blades, Science 295, 1861 (2002).
(7) W.L. Shoop, Y. Xiong, J. Wiltsie, A. Woods, J. Guo, J.V. Pivnichny, T. Felcetto, B.F. Michael, A. Bansal, R.T. Cummings, B.R. Cunningham, A.M. Friedlander, C.M. Douglas, S.B. Patel, D. Wisniewski, G. Scapin, S.P. Salowe, D.M. Zaller, K.T. Chapman, E.M. Scolnick, D.M. Schmatz, K. Bartizal, M. MacCoss, and J.D. Hermes, Proc. Nat. Acad Sci. USA 102 (22), 7958–796363 (2005).
(8) T.R. Covey, E.D. Lee, and J.D. Henion, Anal. Chem. 58, 2453–2460 (1986).
(9) E.D.Lee, W. Muck, J.D. Henion, and T.R. Covey, Biomed Environ. Mass Spectrom. 18, 253–257 (1989).
(10) I. Fu, E.J. Woolf, and B.K. Matuszewski, Pharm. Biomed. Anal. 18, 347 (1998).
(11) M. Groff, K. Riffel, H. Song, and M.W. Lo, J. Chromatogr., B 842, 122–130 (2006).
(12) J. Moreno-Farre, Y. Asad, S. Pacey, P. Workman, and F.I. Raynaud, Rapid Commun. Mass Spectrom. 20, 2845–2850 (2006).
(13) R. King, R. Bonfiglio, C. Fernandez-Metzler, C. Miller-Stein, and T. Olah, J. Am. Soc. Mass Spectrom. 11, 942 (2000).
(14) B.K. Matuszewski, C.M. Chavez-Eng, and M.L. Constanzer, Anal. Chem. 70, 882 (1998).
(15) D. Buhrman, P. Price, and P. Rudewicz, J. Am. Mass Spectrom. 7, 1099–1105 (1996)

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.













