Determination of Pesticide Residues in Marijuana and Tea by QuEChERS and LC–MS–MS
The Application Notebook
UCT, LLC
Xiaoyan Wang, UCT, LLC
This application note outlines a simple method for the rapid determination of pesticide residues in marijuana and tea leaves. Dry samples are hydrated with water followed by QuEChERS extraction and dispersive solid-phase extraction (dSPE) cleanup using UCT's ChloroFiltr®, a specially designed sorbent to selectively remove chlorophyll without affecting the recovery of planar pesticides which can occur when carbon is used for dSPE cleanup. Chlorophyll removal is important as it can adversely impact gas chromatographic (GC) or liquid chromatographic (LC) injector and detection systems.

Extraction and Cleanup Materials
QuEChERS Extraction
1) Weigh 2 g of homogenized tea or marijuana sample into 50 mL centrifuge tubes (RFV0050CT).
2) Hydrate the samples by adding 10 mL of reagent water to the tubes and mix at a low speed for 1 h with a horizontal shaker.
3) Add internal standard to all samples.
4) For fortified samples, add appropriate volumes of pesticide standard solution.
5) Add 10 mL of acetonitrile and vortex for 1 min.
6) Add contents of Mylar pouch (ECQUUS2-MP) to each tube and shake vigorously for 1 min.
7) Centrifuge the samples at 3830 rcf for 5 min.

Figure 1: Extracts (a) before and (b) after dSPE cleanup.
dSPE Cleanup
1) Transfer 1 mL supernatant into a 2-mL dSPE tube (CUMPSGG2CT).
2) Vortex for 30 s.
3) Centrifuge at 15,300 rcf for 5 min.
4) Transfer 0.3 mL of the purified extract into a 2-mL auto-sampler vial.
5) Add 0.3 mL of reagent water, vortex, and then filter using a 0.45 μm syringe filter.
6) The samples are ready for LC–MS–MS analysis.
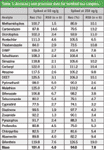
Table 1: Accuracy and precision data for fortified tea samples.
Conclusion
As described, this is a simple, fast, and cost-effective method for the determination of pesticide residues in tea and marijuana samples. After hydrating the samples, pesticide residues were extracted using a non-buffered QuEChERS approach, followed by dSPE cleanup using MgSO4 for water removal; primary secondary amine (PSA) for removing organic acids, sugars, and some pigments; and ChloroFiltr® to selectively remove chlorophyll. The result is a clean extract for LC–MS–MS analysis. Good accuracy and precision were obtained using this method.

UCT, LLC
2731 Bartram Road, Bristol, Pennsylvania 19007, USA
Tel: (215) 781 9255
Email: methods@unitedchem.com
Website: www.unitedchem.com

Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.
A Guide to (U)HPLC Column Selection for Protein Analysis
April 16th 2025Analytical scientists are faced with the task of finding the right column from an almost unmanageable range of products. This paper focuses on columns that enable protein analysis under native conditions through size exclusion, hydrophobic interaction, and ion exchange chromatography. It will highlight the different column characteristics—pore size, particle size, base matrices, column dimensions, ligands—and which questions will help decide which columns to use.














