Characterization of Chitosan by Triple Detection Size Exclusion Chromatography (TD-SEC)
Malvern Instruments Ltd.
Chitosan samples from different sources are fully characterized for molecular weight, intrinsic viscosity, and molecular structure by triple detection size exclusion chromatography (TD-SEC).
The linear polysaccharide chitin is one of the most abundant natural materials in the world and composes most of the exoskeleton of anthropods and the radula of molluscs. Chitin is insoluble in water but once deacetylated, the resulting form, chitosan, can be dissolved in acetic acid. Chitosan is bio-degradable over time, making it an inexpensive and abundant eco-friendly material.
Chitosan Samples
Six chitosans from various sources were obtained (1) and the degree of acetylation was determined by 1H-NMR measurements as explained in reference 2. The sample information is shown in the table.
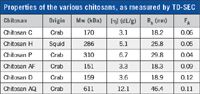
Properties of the various chitosans, as measured by TD-SEC
Experimental Conditions
Triple detection size exclusion chromatography (TD-SEC) was performed at 30 °C using a Viscotek TDAmax system, two Viscotek ViscoGEL columns, and a mobile phase consisting of 0.3 M acetic acid and sodium acetate buffer at 0.7 mL/min.
The chitosan samples were prepared at 0.3 to 1 mg/mL, dissolved for 24 h under light agitation, and then filtered using a 0.45 μm filter prior to injection.
Results
The data is separated into two groups of three. The first three samples have a comparable fraction of acetylaction of about 0.05 and the second group around 0.10 but show different molecular weights.
Using TD-SEC, the intrinsic viscosity and the molecular weight were measured for each elution slice. The Mark-Houwink plot (M-H plot) is given for all the chitosan samples in Figure 1. For molecular weights below 170 kDa, the slope of the M-H plot is around 1.0 for all chitosans, indicating similar densities or coiling. The difference in slope is noted for molecular weights above 170 kDa where there is a curvature in the M-H plot. The change of slope is due to a change to tighter polymer coiling at high molecular weight.
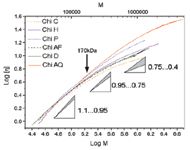
Figure 1: Mark-Houwink plot for all the chitosan samples.
Conclusion
Accurate molecular weights and molecular weight distributions were measured using triple detection. The degree of acetylation did not correlate with the molecular weight, inferring that it is possible to have highly acetylated chitosan with low or high molecular weights. The viscometer was used to correlate the molecular weight to the size of the chitosan, and this proved that the use of Ubbelhode-type viscometric measurements to estimate the molecular weight are flawed as the M-H curve exhibits a curvature.
References
(1) Application Note MRK1295-01, Malvern Instruments Knowledgebase article.
(2) M.X. Weinhold, J.C.M. Sauvageau, N. Keddig, M. Matzke, B. Tartsch, I. Grunwald, C. Kübel, B.Jastorff, and J. Thöming, Green Chem. 11, 498 (2009).

Malvern Instruments Ltd.
Enigma Business Park, Grovewood Road,Malvern, UK
Tel: +44 (0) 1684 892456
Website: www.malvern.com

Determining the Link Between Prenatal Cannabis Use and Symptoms of Depression Using LC–MS/MS
April 16th 2025Researchers investigating the relationship between cannabis use during pregnancy and depressive symptoms—and whether continued use beyond the first trimester or higher levels of use were linked to increased symptoms—used liquid chromatography–tandem mass spectrometry (LC–MS/MS) to confirm the presence of 11-nor-9-carboxy-delta-9-tetrahydrocannabinol (THC-COOH) in urine samples.
A Guide to (U)HPLC Column Selection for Protein Analysis
April 16th 2025Analytical scientists are faced with the task of finding the right column from an almost unmanageable range of products. This paper focuses on columns that enable protein analysis under native conditions through size exclusion, hydrophobic interaction, and ion exchange chromatography. It will highlight the different column characteristics—pore size, particle size, base matrices, column dimensions, ligands—and which questions will help decide which columns to use.
Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.














