Fast Extraction of Metanephrine and Normetanephrine from Urine Using EVOLUTE® EXPRESS CX Prior to LC–MS–MS Analysis
The Application Notebook
Biotage LLC
Frank Kero and Victor Vandell, Biotage LLC
A new solid-phase extraction (SPE) technology has been developed to speed up sample preparation by eliminating steps in the extraction workflow. This application note describes the extraction of metanephrine and normetanephrine from synthetic urine using this 'load-wash-elute' approach with EVOLUTE EXPRESS CX 96-well SPE plates. Recoveries exceeding 80% and excellent linearity over the range 10–70 ng/mL are achieved.
Extraction Conditions
Plate configuration: EVOLUTE EXPRESS CX 30 mg plate p/n 601-0030-PX01
Sample pre-treatment: Dilute synthetic urine sample with water (1:1, v/v).
Sample loading: Load pre-treated sample (1 mL) at a flow rate of 1 mL/min using positive pressure (PRESSURE + 96 Positive Pressure Manifold, p/n PPM-96). Note that plate conditioning and equilibration steps are not required.
Interference wash 1: Water (1 mL)
Interference wash 2: Methanol (1 mL)
Analyte elution: Methanol–concentrated ammonium hydroxide (95:5, v/v, 1 mL).
Post extraction: Evaporate extracts to dryness (SPE Dry Dual Sample Concentrator, p/n SD-9600-DHS) and reconstitute in 80/20 (v/v) mobile phase A–mobile phase B (500 μL).
HPLC Conditions
Instrument: Agilent 1200 Liquid Handling System (Agilent Technologies, Berkshire, UK)
Column: Organic Acids 150 mm × 4.6 mm (5 μm) (Restek)
Mobile phase A: 1% (v/v) formic acid in water
Mobile phase B: Acetonitrile
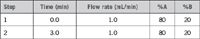
Gradient:
Mass Spectrometry Conditions
Instrument: Applied Biosystems/MDS Sciex 4000 Q-Trap triple quadrupole mass spectrometer (Applied Biosystems, Foster City, California, USA) equipped with a Turbo Ionspray interface.
Monitored transitions: Metanephrine 198>165; normetanephrine 185>166.
Results
Figure 1 shows the extracted ion chromatogram for metanephrine and normetanephrine from a 20 ng/mL fortified synthetic urine sample. The method demonstrated high (>80%), reproducible recoveries of both analytes, with excellent linearity (r2 >0.99) over the range 10–70 ng/mL.

Figure 1: Extracted ion chromatogram for metanephrine and normetanephrine (20 ng/mL fortified urine sample).
Conclusions
The load-wash-elute procedure utilizing EVOLUTE EXPRESS CX plates described in this application note proved fast, simple, and effective for the extraction of the catecholamine metabolites, metanephrine and normetanephrine, from synthetic urine samples. Elimination of the traditional conditioning and equilibration steps speeds up the sample preparation process, without compromising on analytical quality.
Visit the literature database on biotage.com for more information.

Biotage AB
Vimpelgatan 5, Uppsala, Sweden
Tel: +46 18 56 59 00 fax: +46 18 59 19 22
Email: info@biotage.com
Website: www.biotage.com

Determining the Effects of ‘Quantitative Marinating’ on Crayfish Meat with HS-GC-IMS
April 30th 2025A novel method called quantitative marinating (QM) was developed to reduce industrial waste during the processing of crayfish meat, with the taste, flavor, and aroma of crayfish meat processed by various techniques investigated. Headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS) was used to determine volatile compounds of meat examined.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)












