Peptide Mapping of Glycoprotein Erythropoietin by HILIC LC–MS and RPLC–MS
The Application Notebook
Agilent Technologies, Inc
Alex Zhu, James Martosella, and Phu T. Duong, Agilent Technologies Inc.
Peptide mapping is an important technique for the comprehensive characterization of protein biotherapeutics. Reversed-phase ultrahigh-pressure liquid chromatography/high performance liquid chromatography (UHPLC/HPLC) is routinely used, but if the digest contains hydrophilic peptides, valuable information can be missed. In this work, we demonstrate peptide mapping of digested glycoprotein erythropoietin (EPO) using hydrophilic interaction chromatography (HILIC) as a complementary approach to reversed-phase chromatography peptide analysis. An Agilent ZORBAX Rapid Resolution High Definition (RRHD) 300-HILIC 1.8 μm LC column and AdvanceBio Peptide Mapping RP column, in combination with time-of-flight (TOF) mass spectrometry (MS), were used for mapping EPO protein. Taking advantage of the high organic solvent system of the mobile phase for HILIC, the digested peptides from these analyses were evaluated and compared for sequence coverage and peptide identification. This work demonstrates the utility of HILIC as an orthogonal and complementary approach to reversed-phase LC–MS for peptide analysis.
Results and Discussion
The elution order in reversed-phase LC and HILIC is orthogonal. In reversed-phase separation, the digested peptides from EPO protein are eluted in order of increasing hydrophobicity, but with HILIC the least hydrophobic peptides (hydrophilic) will be retained most strongly by the column. Subsequently, the elution order is reversed. The use of HILIC columns for the analysis of the peptides obtained from an enzymatic digest of a protein would therefore be expected to provide increased retention and resolution of the hydrophilic peptides, including glycopeptides, compared with reversed-phase columns. Hence, digested peptides can be identified by HILIC that may not have been retained and resolved by reversed-phase chromatography.
The biotherapeutic glycoprotein, EPO, is a small protein and has a molecular weight of approximately 34,000 Da. It is known to be heavily glycosylated and therefore a tryptic digest would be expected to contain a range of peptides, including hydrophilic peptides and glycopeptides.
A comparison of mass spectrometry analysis of digested peptides from EPO glycoprotein using ZORBAX RRHD 300-HILIC and AdvanceBio Pepping Mapping RP column is shown in Figure 1a and 1b.

Figure 1: (a) Extracted compound chromatograms of matched erythropoietin (EPO) digested peptides from an Agilent ZORBAX RRHD 300-HILIC column and (b) an Agilent AdvanceBio Peptide Mapping RP column, both using the Agilent MassHunter molecular feature extractor.
The HILIC LC–MS results were extracted using the Agilent MassHunter molecular feature extractor and then matched to the digested EPO protein sequence, showing that the sequence coverage was 100% (Figure 1a). It is noteworthy that the separation took less than 15 min.
The same sample was then analysed using the AdvanceBio Peptide Mapping RP column. Extracted compounds of matching EPO-digested peptides again showed 100% sequence coverage (Figure 1b).
Peptides Common to HILIC and Reversed-Phase
Eight peptides were present from both columns when the data were compared. This indicated that these peptides had affinity for both modes of chromatography (see Table 1). Generally, the elution order of the HILIC profile will be opposite to that of the reversed-phase profile, but not always. Elution orders are dictated by hydrophobicity and charge (on the peptides). Therefore, the HILIC order does not necessarily go from 8 to 1 as shown in Figure 2.
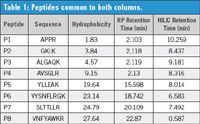
Table 1: Peptides common to both columns.
Peptides P1 to P4 were resolved better with the ZORBAX RRHD 300-HILIC column, as shown in the top and bottom panels of Figure 2. They eluted together on the reversed-phase column.

Figure 2: Comparing eight peptides from both columns for their retention times and resolutions.
Peptides Found Only from HILIC
Generally, under reversed-phase conditions, the least hydrophobic peptides (hydrophilic) will elute early, making their quantitation by MS analysis more difficult. Moreover, some very hydrophobic peptides are difficult to dissolve in aqueous conditions, which are usually used as solvents for reversed-phase LC–MS analysis. This also leads to lower sensitivity. Therefore, with high organic solvent mobile phase, and the sample mixed with a high percentage organic solvent, some highly hydrophobic peptides will be dissolved and separated better with the HILIC column. Data from Table 2 provides an example showing hydrophilic peptides that are only be identified by HILIC LC–MS.
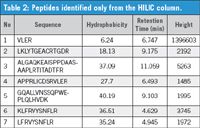
Table 2: Peptides identified only from the HILIC column.
Glycopeptides from Trypsin-Dgested EPO Protein Found from HILIC Column
From the data in Table 2, peptide number 5 is the glycopeptide found only from the HILIC column. Its sequence location, retention, and glycosylation identification are indicated in Table 3. Moreover, one additional glycopeptide with the sequence EAENITTGCAEHCSLNENITVPDTK was identified using ZORBAX RRHD 300-HILIC column and has four different glycoforms (Table 3).
EPO glycoprotein has three N-glycosylation sites at sequence locations 24, 38, and 83. The glycopeptides identified by the HILIC column are listed in Table 3 with the glycosylation sites presented in red colour. Based on their retention times of the MS chromatogram (Figure 1a), these glycopeptides are very hydrophilic peptides. Similarly, four glycoforms of glycopeptide EAENITTGCAEHCSLNENITVPDTK were identified by the reversed-phase column. However, these glycoforms were eluted in void volume of the reversed-phase column without being resolved.
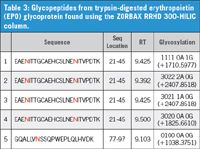
Table 3: Glycopeptides from trypsin-digested erythropoietin (EPO) glycoprotein found using the ZORBAX RRHD 300-HILIC column.
Experimental
Sample Preparation
A sample of trypsin-digested EPO glycoprotein was purchased from Bio Creative, Shirley, New York, USA; 100 μL of the sample (2 mg/mL) was mixed with 100 μL of HILIC or reversed-phase eluent A solvent, as appropriate.
Operating Conditions
Experiments were performed on an UHPLC–TOF system, comprising an Agilent 1290 Infinity LC, accurate-mass 6224 TOF-LC–MS, with dual electrospray ionization (ESI) source in positive mode. Peptides from trypsin-digested EPO protein were separated using different HILIC and reversed-phase conditions.
Conditions, HILIC
Columns: Agilent ZORBAX Rapid Resolution High Definition 300-HILIC, 2.1 × 100 mm, 1.8 μm (p/n 858750-901)
Eluent: A = 95% acetonitrile + 5% water; B = 50 mM ammonium formate, pH 4.0
Flow rate: 0.4 mL/min

Conditions, Reversed-phase
Column: Agilent AdvanceBio Peptide Mapping, 2.1 × 250 mm, 2.7 μm (p/n 653750-902)
Eluent: A = 100% water, 0.1% formic acid; B = 100% acetonitrile, 0.1% formic acid
Flow rate: 0.4 mL/min

MS Conditions
Gas temperature: 350 °C
Gas flow: 10 L/min
Nebulizer: 45 psi
Capillary voltage: 3500 V
Fragmentor: 170 V
Scan rate: 2 spec/s
Mass range: 400–3200 m/z
Conclusions
The use of ZORBAX Rapid Resolution High Definition 300-HILIC could aid the mapping and identification of hydrophilic peptides that were not resolved by reversed-phase chromatography. Therefore, coupling this column with MS could be an orthogonal and complementary approach to reversed-phase LC–MS, to provide better retention for hydrophilic peptides including glycopeptides, therefore, potentially providing better sequence coverage and protein characterization.

Agilent Technologies Inc.
2850 Centerville Road
Wilmington, Delaware 19808, USA

Pick Your Poison. Isolation of Paclitaxel (Mar 2025)
March 7th 2025The diterpenoid, paclitaxel, which was identified as a potent chemotherapy agent for breast and ovarian cancer originates from the Pacific Yew tree. The isolation of paclitaxel from its major impurities is shown with the use of Hamilton’s PRP-1 (5 µm) HPLC column.










