Determination of Genotoxic Besylates and Tosylates by HPLC-ECD Using A Boron-Doped Diamond Electrode
Genotoxic impurities in pharmaceutical products have come under increasing regulatory scrutiny, with exposure limits <1.5 μg/day typically being reported for most products.
Marc Plante, Bruce Bailey, and Ian Acworth, ESA - A Dionex Company, Inc.
Genotoxic impurities in pharmaceutical products have come under increasing regulatory scrutiny, with exposure limits <1.5 μg/day typically being reported for most products. Techniques for accurate quantitation of these impurities in active pharmaceutical ingredients (APIs) are limited. Two classes of compounds that can be found in synthetically produced APIs include known genotoxic compounds–besylates and tosylates– used as protecting groups during a synthetic process. The analysis of these genotoxic compounds requires significant sensitivity because they often exist at ppm levels compared to the API.
HPLC with electrochemical detection (HPLC-ECD) is an extremely sensitive technique and has been used previously to measure the genotoxic aminopyridines (1), and DNA adducts formed from the interaction between nucleic acids and a genotoxic material (2,3). Using a boron-doped diamond (BDD) working electrode at high potential (+2000 mV), ECD can detect tosyl or besyl compounds to 400 pg on column. The mechanism is believed to involve the formation of hydroxyl-free radicals, their attack of the benzene ring, and EC monitoring of the resulting phenol (4).
Experimental
EC-compatible HPLC system with a Coulochem® III Electrochemical Detector, a degasser, a BDD ECD cell, and guard cell; sodium perchlorate (Fluka), perchloric acid (GFS Chemicals), hydrogen peroxide (30%; Sigma Aldrich).
HPLC Parameters:
Column: Halo™ C18, 3 × 75 mm, 2.7 μm
BDD Cell and Column Temp.: 35 °C
Sample Temp.: 10 °C
Mobile Phase: 40 mM sodium perchlorate,
20 mM perchloric acid,
980 μM H2O2 in water/acetonitrile (65:35)
Flow Rate: 0.45 mL/min
Injection Vol.: 10 μL
Guard Cell: +1000 mV (vs. Pd reference)
BDD Cell: +2000 mV for besylates (vs. Pd reference)
+1900 mV for tosylates (vs. Pd reference)
Filter: 5 s
Results
An example of the analysis of methyl and ethyl besylate and methyl tosylate is seen in Figure 1. A small amount of H2O2 provides the radicals needed for the analysis of besylates. Calibration curves exhibited coefficients > 0.999. The LOD values were ≤ 190 pg for tosylates and < 400 pg on column for besylates.
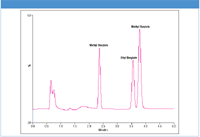
Figure 1: Analysis of besylate and tosylate genotoxins using BDD working electrode at +2000 mV (~6 ng each on column).
Conclusion
A simple, reliable, and sensitive ECD-based method is presented here for the measurement of two classes of genotoxic impurities (alkyl tosylates and alkyl besylates) with LODs of 190 and 400 pg on column, respectively. The assay has the sensitivity required for routine detection of these impurities in a pharmaceutical formulation with a linear response over three orders of magnitude.
References
(1) ESA Application Note 70-8311, Aminopyridines by BDD.
(2) Park, J-W; Cundy, K.C.; Ames, B.N. Carcinogenesis, 10 (5), 827–832 (1988).
(3) Wessela, N.; Rousseaua, S.; Caiseya, X.; Quinioua, F.; Akcha, F. Aquatic Toxicology, 85 (2), 133–142 (1988).
(4) U.S. Patent Application 20070193886, Detection Methods and Devices, Acworth, I.; Gamache, P.; Granger, M.
Coulochem is a registered trademark of Dionex Corporation.
Halo is a trademark of Advanced Materials Technology, Inc.
ESA – A Dionex Company
22 Alpha Road, Chelmsford, MA 01824
tel. (978) 250-7000; fax (978) 250-7087
Websites: www.esainc.com; www.dionex.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.















