Chiral Analysis and Purification Using Kromasil CelluCoat
LCGC Asia Pacific
The necessity to analyse and purify chiral compounds has increased. This has generated demand for chiral stationary phases (CSP) with good chiral selectivity and high loadability. Kromasil CelluCoat is a silica based CSP coated with tris-(3,5-dimethylphenyl)-carbamoyl cellulose as the chiral selector. The chiral interaction between analyte and a CSP like CelluCoat in normal phase mode is a combination of steric, H-bond, π–π, and dipole interactions.1
This new coated phase is a great addition to the existing immobilized CSP's from Kromasil, namely TBB and DMB.2
Analytical separation
Two of the most predominant factors in analytical chiral HPLC are selectivity leading to enantiomer separation, and hereafter the speed of the separation. For analytical separations it is ideal to use small particle CSP, which gives high chromatographic resolution and makes it possible to run fast separations at high flow-rates without loosing significant column efficiency. Kromasil CelluCoat withstands flow-rates equivalent to pressures up to 400 bar.
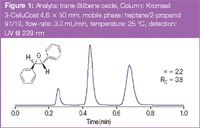
Figure 1
In Figures 1 and 2 the analytical separations of racemic trans-stilbene oxide and propranolol a β-blocker, are displayed.
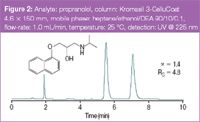
Figure 2
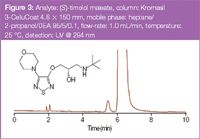
Figure 3
As demanded by the Food and Drug Administration (FDA) chiral pharmaceuticals should be developed and reach the market as single enantiomer products.3 This fact makes robust chiral separation methods essential for product quality control analysis, because the unwanted enantiomer is often the most critical product impurity. Figure 3 shows a purity analysis of the β-blocker (S)-timolol maleate. The (R)-enantiomer is seen in front of the main peak.
Preparative purification
In the field of chiral chromatography preparative purification needs range from semi-prep purification in the lab during early development to full scale manufacturing of enantiopure drugs.4
Figure 4 is a chromatogram showing the semi-preparative separation of the β-blocker metoprolol. With a load of 4.8 mg racemate per gram of stationary phase touching bands are obtained, leading to full recovery of pure enantiomers.
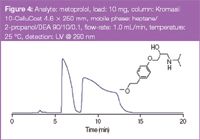
Figure 4

Figure 5
True preparative chiral chromatography is performed by pushing the CSP to its maximum loading capacity. By doing so possible displacement effects can be utilized, leading to increased productivity of the purification process. Figure 5 shows an overlay of a preparative chromatogram and the elution profiles of the enantiomers reconstructed from fraction analysis data. In this instance 80% of the feed was recovered with 90% enantiomeric purity, which is a good starting point for reaching >99% ee after recrystallization. It should, however, be mentioned that competing isotherms are not the case for all chiral separations.
Conclusion
This short paper presents Kromasil CelluCoat as a versatile CSP that performs well in all areas of chiral chromatography. Kromasil CelluCoat is a good choice for fast and robust analytical separations as well as for preparative enantiomer purification both in the laboratory and in full scale production. Kromasil CelluCoat is available in 3 μm, 5 μm and 10 μm particle size packed in stainless steel columns. In addition 10 μm Kromasil CelluCoat is available as bulk material.
Britt Kofoed-Hansen, Akzo Nobel, Bohus, Sweden.
References
1. R. Thompson, J. Chromatogr & Rel. Techn., 28, 1215–1231 (2004).
2. S.G. Allenmark et al., Chirality, 7(4), 248–256 (1995).
3. FDA, Chirality, (1992).
4. E.R. Francotte, J. Chromatogr. A, 906, 379–397 (2001).
Kromasil and CelluCoat are registered trademarks of Akzo Nobel.

Akzo Nobel
Eka Chemicals AB
Separation Products
SE-445 80 Bohus, Sweden
tel. +46 31 58 73 60 fax. +46 31 58 77 27
E-mail: Kromasil@eka.com Website: www.kromasil.com

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)













