Analysis of PFOA and PFOS in Water Using Reversed-Phase HPLC with Suppressed Conductivity Detection
LCGC Asia Pacific
Suppressed conductivity detection is a well-developed method for detecting charged species. Reversed-phase high-performance liquid chromatography (RP-HPLC) is a well developed method of separating substances on the basis of hydrophobicity. There are some situations where it is advantageous to use these two methods together. Perfluoro-acids (PFOAs) are one class of compounds that are ionic, hydrophobic and have low UV absorbance and are, therefore, suited to this combination.
Suppressed conductivity detection is a well-developed method for detecting charged species. Reversed-phase high-performance liquid chromatography (RP-HPLC) is a well developed method of separating substances on the basis of hydrophobicity. There are some situations where it is advantageous to use these two methods together. Perfluoro-acids (PFOAs) are one class of compounds that are ionic, hydrophobic and have low UV absorbance and are, therefore, suited to this combination.
PFOA and perfluorooctanesulphonic acid (PFOS) are medium-chain perfluorinated acids with an assortment of industrial and consumer uses. They have been implicated in various health issues and are widely distributed and persistent in the environment. In this application, PFOA and PFOS in drinking water are concentrated by a column switching valve onto a cartridge, separated by RP-HPLC on an Acclaim PA2 column and detected by suppressed conductivity.
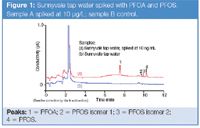
Figure 1
Conditions
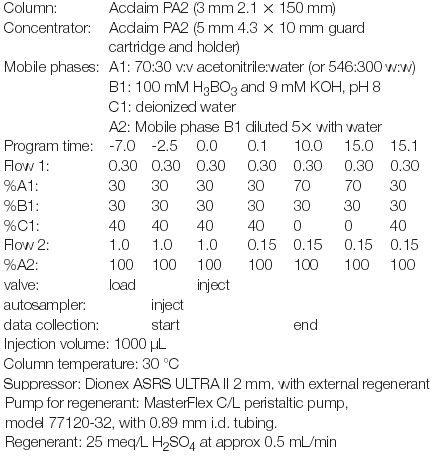
HPLC system: Dionex ICS-3000 configured with DP dual gradient pump; DC detector/chromatography module equipped with conductivity detector and 6-port switching valve; ASI-100 autosampler equipped with 1000 μL syringe and loop. Pump 1 connects to port 3 on the switching valve and it provides the elution gradient. Pump 2 connects to the autosampler and loads the sample onto the concentrator and washes it. The autosampler outlet connects to port 6 of the valve. The concentrator is connected to ports 1 and 4, replacing the sample loop. The analytical column connects to port 2. Port 5 goes to waste through a restrictor capillary. When the valve is in the load position, the sample is injected onto the concentrator, which is washed by pump 2 to remove matrix interferences. It is switched to the inject position for elution by pump 1.
Results and Discussion
We selected a borate buffer at pH 8 because of its low background conductivity. The separation was performed on an Acclaim PA2 column because it provides excellent resistance to alkaline conditions and better loadability compared to standard RP columns (C18). Since the detection limit by conductivity is around 0.5 ng, to achieve low detection limits, we used a preconcentration technique, as described above.
Because fluorocarbon polymers are frequently used in HPLC instruments, they present two difficulties for analysing fluorinated organics: carry-over and sample adsorption. We selected the Summit ASI-100 autosampler to greatly minimize these problems because its sample-wetted surfaces are PEEK and stainless steel.
Figure 1 shows typical chromatography for the analysis of a tap water sample. Spike recoveries between 2 and 200 μg/L were 98–101% for PFOA and 97–137% for PFOS. Similar results were obtained for bottled water and a synthetic water matrix.
Conclusion
We report a detection limit of 0.5 μg/L using an RP HPLC/suppressed conductivity detection method for analysing PFOS and PFOA. This technique can be applied to other fluorinated organic acids (C6–C18 homologs) as well as a variety of surface active sulphates and sulphonates (e.g., SDS, LAS, etc.).

Dionex Corporation
1228 Titan Way, P.O. Box 3603, Sunnyvale, California 94088-3603, USA
tel. +1 408 737 0700 fax +1 408 730 9403 Website: www.dionex.com

Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.


















