2D Analysis of Thermoplastic Elastomers TPE
LCGC Asia Pacific
Polymer Standard Services
Introduction
Blockcopolymers such as SBS (styrene-butadiene-styrene) are an important product class and a typical example of complex polymers. In addition to the molar mass distribution, a chemical composition distribution may also be present in copolymers. While GPC/SEC is the established method for the determination of molar mass averages and distribution, gradient HPLC can be applied to separate based on chemical composition.
Gradient HPLC can be hyphenated with GPC/SEC in a fully automated setup to measure both distribution simultaneously with a high peak capacity and to detect differences in batches (cf. Figure 1).

Figure 1: Scheme of two-dimensional chromatography.
Experimental
All experiments were performed on PSS SECcurity equipment using the following conditions:
Eluent 1st dim.: n-Hexane/THF p.a. gradient
Column 1st dim.: PSS Si-60 5 μm
Flow-rate 1st dim.: 0.1 mL/min
Injection volume: 20 μL
Transfer: PSS 2D tandem transfer valve with two 100 μL loops
Eluent 2nd dim.: THF p.a.
Column 2nd dim.: PSS HighSpeed SDV 5 μm, 10000 Å
Flow-rate 2nd dim.: 6.25 mL/min
Detection: SECcurity VWD 1260 UV at 254 nm
Calibration: PSS Polystyrene ReadyCal Standards, PSS Polybutadiene standards
Data system: PSS WinGPC Unity 7.5
Results
The 2D approach is the only way to determine two property distributions independently and unambiguously. The online combination of gradient HPLC and GPC/SEC increases the peak capacity of the separations and those peaks which cannot be separated by either method alone to be examined more closely. The HPLC conditions are selected to separate according to polybutadiene content.
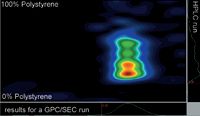
Figure 2: Contour plot of a thermoplastic elastomer.
Figure 2 shows the contour plot for a thermoplastic elastomer that shows one narrow main peak in GPC/SEC. However, 2D separation reveals that four different compositions are present that co-elute in the GPC/SEC experiment. The species differ in composition and polybutadiene content. The colour code indicates the concentration of each peak. Simultaneous molar mass results and composition results can be measured using the calibrated GPC/SEC and HPLC axis.
PSS Polymer Standards Service GmbH
In der Dalheimer Wiese 5, D-55120 Mainz, Germany
tel.+49 6131 96239 0 fax +49 6131 96239 11
E-mail: Pkilz@polymer.de
Website: www.pss-polymer.com

A Novel LC–QTOF-MS DIA Method for Pesticide Quantification and Screening in Agricultural Waters
May 8th 2025Scientists from the University of Santiago de Compostela developed a liquid chromatography quadrupole time-of-flight mass spectrometry (LC–QTOF-MS) operated in data-independent acquisition (DIA) mode for pesticide quantification in agriculturally impacted waters.
Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)













