Ultratrace Quantitive Analaysis of Catalyst Poisoners Using a Dedicated GC–MS Analyser
LCGC Asia Pacific
A dedicated GC– MS analyser was developed to address the increasing need for more sensitive catalyst poisoner analysis. The system combines the separation power and robustness of a classic backflush configuration with the selectivity and sensisitivitiy of mass spectrometry.
A dedicated GC–MS analyser was developed to address the increasing need for more sensitive catalyst poisoner analysis. The system combines the separation power and robustness of a classic backflush configuration with the selectivity and sensitivity of mass spectrometry.
The use of high-yield metallocene catalysts has dramatically increased both efficiency and selectivity of polymerization processes (1). Unfortunately, these catalysts are extremely prone to poisoning by feedstock impurities, such as arsine (AsH3), phosphine (PH3), oxygenates (for example, dimethylether) and sulphur-containing compounds (mercaptanes, sulphides, etc) (2,3). Minute amounts of these compounds are sufficient to impose undesirable effects and induce immediate loss of catalytic activity and reaction yield. At the same time, trace contaminants at the part-per-billion (ppb) concentration levels can end up in the polymers and alter subsequent polymer properties and characteristics.

For decades, process chemical and petrochemical analysts used to address their analytical challenges mainly by relying on superior chromatography and smart tools such as valve switching, backflush and Dean's heart-cut. In combination with relatively cheap, robust and selective detectors, they were capable of providing all information necessary to control and tweak petrochemical processes.
Organic catalyst poisoners are usually determined using dedicated chromatographic analysers. These systems are, typically, equipped with a dual capillary column configuration with backflush and fitted with a flame ionization detector (FID). Under these conditions, limits-of-detection are usually situated around 100 ppb, depending on the compound investigated and the complexity of the matrix that is introduced (4). Unfortunately, this is far from sufficient to protect the latest catalysts, which start to deteriorate as soon as fed with low ppb amounts (5,6). An overview of some typical specifications for catalyst poisoners in polymer-grade hydrocarbons is given in Table 1.
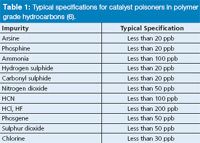
Table 1: Typical specifications for catalyst poisoners in polymer grade hydrocarbons (6).
Mass spectrometry (MS) is hardly used in petrochemical QC laboratories, which is primarily because of its apparent complexity and higher cost-of-ownership. Nonetheless, MS detection has several distinct advantages over classic analogue detectors. In full scan acquisition mode, for example, it allows tracking and identification of unknown components using spectral deconvolution and subsequent library matching. In selective ion monitoring (SIM) mode, on the contrary, MS permits trace and ultratrace quantification of target analytes which is often superior to classic selective detectors. Furthermore, MS permits the use of mass labelled internal standards (ISs) that behave identically to their native analogues, which has a positive effect on overall method precision and accuracy. It is no surprise that instrument manufacturers have invested substantially in solutions aimed at reducing overall MS complexity and total cost-of-ownership in the last couple of years. Easy tune and calibration functionalities, increased sensitivity and speed, new acquisition modes and elegant solutions that eliminate downtime, such as vacuum lock technology, have contributed largely in this respect.
This article gives an overview of the main characteristics and performance of a new gas chromatography mass spectrometry (GC–MS) analyser that has been recently developed. The system combines the chromatographic separation power and backflush/Dean's heart-cut capabilities of a classic oxygenate analyser with the orthogonal separation power, sensitivity, selectivity and overall robustness of the latest generation single quadrupole mass spectrometers.
Experimental
Standards: Standard oxygenate reference mixture from Spectrum (Sugarland, Texas, US) at 10 ppm in hexane. For more details with respect to the composition of the test mixture, please consult Table 3. Calibration standards were prepared by gradual dilution in hexane at 0.01 ppm, 0.05 ppm, 1 ppm and 5 ppm.
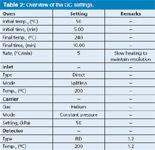
Table 2: Overview of the GC settings.
Gas chromatography: The GC analyser consisted of a Thermo Trace GC (Austin, Texas, US) refurbished by Global Analyser Solutions (GAS, Breda, The Netherlands). The system was fitted with a gas sampling valve (GSV), a liquid sampling valve (LSV), a vapourizer, a standard split/splitless injector and a FID. Inside the GC oven, a universal pressure balanced Deans assembly was installed to carry out heartcut and/or backflush. Auxiliary pressure for balancing was provided and controlled by a separate Trace GC DCC unit.

Table 3: Peak identification and typical SIM ions.
The first dimension column was a Restek Rtx-1 (Bellefonte, Pennsylvania, US) with the following dimensions: 15 mL × 530 µm i.d., 5 µm df. The second dimension column was an Agilent CP-Lowox (Middelburg, The Netherlands) with the following dimensions: 10 mL × 530 µm i.d., 10 µm df. Restrictions were 250 µm i.d. uncoated Siltekdeactived fused-silica capillary tubing (Restek) cut to the appropriate length. All connections were made using micro Siltite unions (SGE Analytical Science Victoria, Australia). Other relevant parameters are summarized in Table 2.
Mass spectrometry: The GC analyser was hyphenated to a Thermo ISQ single quadrupole mass spectrometer. The system was applied in both full scan (range: 15–250 amu) and SIM (dwell time: 0.2 s) as full scan/SIM mode. All relevant MS settings are summarized in Table 3.
All data were acquired using Thermo QuanLab Forms. The MS was applied after running a full EI tune. System performance was verified using a daily tune check.
Results and Discussion
System set-up: The capillary column set comprises the true core of any classic catalyst poisoner analyser. The second dimension column is particularly important. Ultimately, it is here that separation of the analytes, from each other as well as from the aliphatic matrix in which they reside, occurs. A CP-Lowox column (Agilent) was used for this purpose. This column, which is based on a multilayer PLOT concept, is very polar and characterized by a high MAOT with virtually no bleed at temperatures as high as 350 °C (7). In combination with a backflushed apolar-coated capillary column in the first dimension, matrix separations up until C12 hydrocarbons are well within range.
Unfortunately, the CP-Lowox column is not available in an MS-friendly narrow bore dimensions. To avoid the MS vacuum from protruding the system, it is necessary to incorporate an adequate restriction at the back of the column. A schematic representation of system set-up for Lowox/MS applications is depicted in Figure 1.
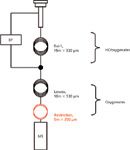
Figure 1: Schematic representation of the GCâMS analyser. BF = backflush; HCs = hydrocarbons.
True backflush, as well as Dean's heart-cut, is achieved by increasing the auxiliary pressure at the column joint just above the first dimension residual head pressure at a certain moment in time. Debalancing induces full flow reversal, whilst maintaining a small flow over the Lowox column for chromatography. It is crucial to know the exact pressure at the column joint for this to be successful. When set too low, standard flow direction will be maintained and the first column is not backflushed. Conversely, when set too high, none of the target analytes will be able to reach the second dimension column and the detector. The easiest way to determine the pressure at the column joint involves setting the head pressure at regular and then reading the residual pressure at the auxiliary DCC, which is kept off at this stage. Five kPa differences are sufficient to induce flow reversal. Although less straightforward because of the vacuum conditions, a similar approach is applied in combination with MS. It also permits the user to determine the minimal length of the restriction capillary (Figure 1).
System suitability: System suitability was evaluated by direct injection of the 10 ppm oxygenate standard. In order to compare with a classic analyser set-up, experiments were performed using both FID and MS as a detector. The MS was applied in both full scan and SIM modes. A typical chromatogram with the MS in full scan mode is depicted in Figure 2. Peak identification is referred to in Table 3.
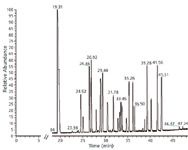
Figure 2: Chromatogram of the oxygenates standard at 10 ppm. The MS was applied in full scan mode. Peak identification is referred to in Table 2.
A comparative overview of the results is given in Table 4. For each peak, the signal-to-noise ratio (S/N) was calculated (RMS) in full scan, extracted ion and SIM mode. These results were subsequently expressed relative to the S/N with FID detection.
The results in Table 4 clearly illustrate the significant gains in sensitivity that can be reached when using MS compared to FID. Minimal gain is 3.7 for ethanol. Unsurprisingly, sensitivity gains are particularly significant when the MS was used in SIM mode. Straight full scan mode proved to be less appropriate for target analysis, which is predominately due to the low molecular weight of the target compounds, and means having to include highly interfering masses such as m/z = 18 (water), 28 (nitrogen) and 32 (oxygen) in the scan range. More convenient in this respect is the combined full scan/SIM mode, which is available on all major instrument brands nowadays.

Figure 3: SIM traces at 0.01 ppm. (a) acetaldehyde; (b) ethanol; and (c) propyl ether.
Afterwards, calibration curves were constructed for each of the oxygenates in the standard mixture from 0.05 ppm to 5 ppm. Correlation coefficients were ≥ 0.995. Some typical SIM traces at the 0.10 ppm level are depicted in Figure 3.
Method repeatability was determined as well. Results at the 10 ppm level are included in Table 4 (six consecutive analyses).

Table 4: Average sensitivity gains in different MS detection modes. FS = full scan; EIC = extracted ion chromatogram; SIM = selected ion monitoring. %RSD at the 10 ppm level (six analyses).
Applications
Naptha feed: Naphtha is a complex mixture of hydrocarbons (C5–C12) in petroleum boiling between 30 °C and 200 °C. Oxygenates are routinely determined in these samples according to reference procedures such as ASTM D7423 (4) as their cracking product cause problems in the downstream separation processes (8). Naphthas are very complex and fully require the chromatographic separation power of the Lowox column. A typical chromatogram of a naphtha sample in SIM mode is depicted in Figure 4. Individual samples were introduced using the LSV of the GC–MS analyser. The insert shows the methanol trace (ion 29, 0.18 ppm); concentrations of acetaldehyde and TAME were 8.3 and 4.9 ppm, respectively.

Figure 4: Typical SIM trace of a naphtha feed.
When idle, the GC oven was kept at 200 °C with the backflush activated. This was necessary to prevent the accumulation of siloxane bleed from the precolumn.
Conclusions
A GC–MS analyser is described that substantially expands the workable application range of a classic catalyst contaminants analyser. The use of mass spectrometry in FS/SIM mode permits identification of unknown contaminants in combination with reliable quantification at trace and ultratrace amounts.
Acknowledgement
The authors acknowledge the financial support from the Long Term Structural Methusalem Funding by the Flemish Government – grant number BOF09/01M00409. KVG holds a Postdoctoral Fellowship of the Fund for Scientific Research Flanders and a BOF tenure track position at Ghent University.
Kevin M. Van Geem is a Fulbright alumnus of the Massachusetts Institute of Technology, Massachusetts, USA, and assistant professor in thermochemical reaction engineering in the department of chemical engineering and technical chemistry at Ghent University, Belgium.
Jeroen Ongenae is a masters student in civil engineering in the department of chemical engineering and technical chemistry at Ghent University, Belgium. He is currently finishing his thesis on trace oxygenate analysis using mass spectrometric techniques.
Jean-Louis Brix is an analytical support engineer at IS-X. He specializes in instrument design and method development for petrochemical applications.
Joeri Vercammen is managing expert of IS-X. He specializes in method development and rationalization from prep-to-rep, method validation and quality assurance.
Guy B. Marin is chair of the department of chemical engineering and technical chemistry at Ghent University, Belgium. Chemical reaction engineering, catalysis in general and reaction kinetics are the main topics in his research programme.
References
(1) L. Resconi, L. Cavallo, A. Fait and F. Piemontesi, Chem. Rev., 100, 1253–1345 (2000).
(2) Almatis AC Inc., Application for Selective Adsorbents in Polymer Production Processes, Technical Bulletin USA/6040-R00/0504.
(3) M.A. Graham, Selected Ethylene Feedstock Impurities: Survey Data. Ethylene Producers Conference, Houston, Texas, USA (1993).
(4) J.G. Speight, Handbook of Petroleum Product Analysis, John Wiley & Sons (2002).
(5) B. Biela, R. Moore, R. Benesch, B. Talbert and T. Jacksier, Gulf Coast Conference, Galveston, Texas, USA (2003).
(6) S.S. Thind, Petro Industry News, 1–2, June/July, (2003).
(7) J. de Zeeuw and J. Luong, Trends Anal. Chem., 21, 594–607, (2002).
(8) S.P. Pyl, C.M. Schietekat, M.-F. Reyniers, R. Abhari, G.B. Marin and K.M. Van Geem, Chem. Eng. J., 176–177, 178–187 (2011).

Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.
University of Oklahoma and UC Davis Researchers Probe Lipidomic Profiles with RP-LC–HRMS/MS
May 6th 2025A joint study between the University of Oklahoma Health Sciences Center (Oklahoma City, Oklahoma) and the UC Davis West Coast Metabolomics Center (Davis, California) identified differentially regulated lipids in type 2 diabetes (T2D) and obesity through the application of reversed-phase liquid chromatography-accurate mass tandem mass spectrometry (RP-LC-accurate MS/MS).
Automated Sample Preparation (ISO 20122) for MOSH/MOAH in Seasoning Oils
May 6th 2025This work presents an Automated Sample Preparation procedure for MOSH/MOAH analysis of Seasoning Oils. We compare results from a manual epoxidation procedure compliant with DIN 16995 with results based on fully automated sample preparation (epoxidation and saponification) compliant with ISO 20122. In both cases, online clean-up via activated aluminum oxide (AlOx) are used to remove interfering n-alkanes from the MOSH fraction during the HPLC run. Automated data evaluation using a dedicated software (GERSTEL ChroMOH) is presented.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)













