Why All C18 SPE Phases Are Not Equal
LCGC North America
Octadecyl bonded silica gel (C18) is widely used in solid-phase extraction (SPE), but there are many variations including particle size, pore size, surface chemistry, and carbon loading applications.
Octadecyl bonded silica gel (C18), available from many manufacturers, is one of the most widely used phases in solid-phase extraction (SPE) applications. But all C18 is not equivalent. Variations can include particle size, pore size, surface chemistry, carbon loading, and other factors. This article explores these differences and their influence on extraction performance.
Octadecyl bonded silica gel (hereafter referred to as C18) was first introduced in 1960s by Abel (1). Since then, the use of C18 has become well established and is one of the most widely used solid-phase sorbents in analytical chemistry (2). This is true not only for its use in analytical columns in liquid chromatography, but also for solid-phase extraction (SPE) applications as well. C18 is especially popular because of its versatility in sample preparation techniques. It is one chemists turn to first because of its broad utility in the sorption of a variety of analytical compounds and because the sorbent is less selective in the type of analytes it extracts when compared to other bonded phases.
It is sometimes assumed that a solid-phase sorbent from one manufacturer is equivalent to a sorbent from any other supplier and that changing from one source to another is an acceptable practice. This is not the case. The result can mean variation not only in the capture efficiency for specific compounds of interest but the overall polarity of compounds that can be adsorbed. Although C18 bonded phases may be obtained from many sources, substantial variations exist among them (3) as each manufacturer has the latitude to prepare the sorbent in a different manner and with differing physical arrangement of functional groups. Though any C18 phase may be an effective sorbent within the constraints of its own chemistry and any C18 phase may be used in new analytical applications, attempting to change sourcing for an established analytical procedure may cause changes in extraction efficiency. Add to this the variation that exists with the base silica gel support, the extent and uniformity of coverage of the functional groups and variations in endcapping, and one can see there are multiple factors affecting product performance.
There may also be substantial variability between C18 batches due to variations in synthesis techniques. These variations affect critical surface chemistry, which determines retention and selectivity for particular analytes and ultimately affects sorbent performance and reproducibility.
To understand why this is true, we will look at several factors that can make each C18 product unique from the others, and will provide a comprehensive understanding of why such differences exist. These factors include
- particle shape, irregular or spherical
- particle size
- pore characteristics
- metal content of silica gel
- surface chemistry
- organic carbon loading
- lot-to-lot consistency.
Octadecyl Bonded Silica — C18
C18 functions as a reversed-phase adsorbent by providing a high-surface-area active substrate. Analytes are retained or adsorbed by one of several interactions, including hydrogen bonding, van der Waals forces, and the polar character of the silanol groups. C18 is designed to be a nonpolar sorbent but depending on the extent of endcapping (if endcapped) and other factors (discussed later), residual hydroxyl (silanol) moieties through which the octadecyl functional group is normally bonded remain. These polar adsorption sites on the solid phase change the selectivity of the sorbent toward various analyte molecules.
Analytical compounds dissolved in an aqueous media act to partition into the solid phase of the C18 sorbent from liquid media. This partitioning occurs because, in simple terms, the analyte prefers to associate with the chemical characteristics on the surface of the solid in preference to remaining dissolved in the liquid phase.
The affinity of the analyte for the solid C18 is related to the log of the octanol–water partition coefficient of the molecule; that is, the water solubility of the compound of interest. This is a measure of the affinity of the compound for octanol (very nonpolar) versus water, which is in turn related to the organic phase bonded to the solid silica gel support.
This affinity can be expressed mathematically in the following simple equation (3):

For a reversed-phase system, Kd is the distribution coefficient of the solute for the sorbent. CB is the concentration on the bonded phase and CS is the concentration in the solution.
This equation indicates that the capacity of a sorbent is a function of the analyte concentration relative to the concentration of the bonded phase. The actual capacity of the sorbent itself is related to many variations in the chemical and physical parameters of bonded C18s that exist among different brands.
The Solid Support
Silica Gel
The silica gel substrate used in bonding reactions is an amorphous, porous, inorganic solid. It is rigid and does not swell in water or organic solvents in contrast to styrene–divinylbenzene or other polymeric organic solid-phase supports that have this potential. The term "gel" is derived from the fact that the material is a highly cross-linked network inorganic polymer and is considered the polymeric form of silicic acid (2). Silica is found naturally in a partially hydrated crystalline (anhydride) form but is not considered pure enough or hydrated enough for chromatographic or SPE purposes. For this reason, synthetic grades are prepared, allowing for control of well defined characteristics such as pore size and surface area. Synthesis of high purity chromatographic grade silica gels is conducted by several different methods including the polymerization of silicic acid. It also can be prepared by the polymerization of tetra-alkyl orthosilicate in the presence of water and acid as shown in Figure 1 (4).
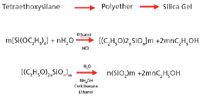
Figure 1: Synthesis of high purity silica gel (4).
The silica support is synthesized to prepare a high surface area material. The resultant gel is a very polar solid because of the surface coverage of free silanol functional groups. The strong polar character of the free silanol groups makes silica gel an excellent desiccant because of its affinity toward highly polar water molecules. It is through these silanol groups that functional carbon groups are bonded. Gels used in SPE have nominal particle sizes of 40 µm with pore sizes on the order of 60 Å. Although the gels are not limited to these values, a 40-µm diameter and 60-Å porosity are the most commonly seen in SPE applications. Base silica gel contains many types of silanol functionality, as shown in Figure 2.
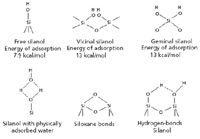
Figure 2: Types of silanol functionality on silica gel (5).
The nature of residual free silanol groups has a direct effect on extraction behavior, because each group has different adsorption bond energy. Though free silanol functionality is by no means a harmful attribute, the nature and extent of the free hydroxyl groups profoundly affect final sorbent performance. Any change in the ratio of free silanol functionality to C18 or endcapped bonded silica affects the type of analytes that are retained on the surface.
Irregular vs. Spherical Silica Particles
Silica gel is prepared as either irregular or spherical particles. The irregular form is obtained by grinding and grading the silica gel to the required size after its synthesis and may range in diameter from 40 to 150 µm. Spherical gel is obtained by spraying a silicate solution into fine droplets before gelling occurs and then drying the droplets in a stream of hot air (6). Spherical particles generally range in size from 5 to 50 µm and have pore diameters that range from about 120 to 300 Å. They are generally more monodisperse than irregular particles and find primary use in high performance liquid chromatography (HPLC) analytical columns. Enhanced performance of separations using spherical silica particles in packed analytical columns has been demonstrated (7). The additional expense required for the preparation of spherical particles and the marginal improvement of performance in SPE applications has largely confined SPE to the use of irregular particles.
Unbonded, pure silica gel is generally considered stable in a pH range of about 2–7.5 (8). Silica gel may be briefly exposed beyond this pH range without deleterious effect if the exposure is relatively short. However, after silica has been covalently bonded with various functional groups, stability is enhanced. Because degradation of silica under acidic or basic conditions is a kinetic process and typical acid contact times during SPE are very short, little if any damage to the gel will result with subsequent interference to the sorptive capability (8). Also, because the solid phases used in SPE techniques are "use-once-then-discard" consumables, any potential harm to the support by extreme pH conditions will not affect subsequent extraction work. This is not true for analytical columns, however, where reproducibility would be seriously affected.
Porosity
The reaction of silicic acid with itself or tetra-alkyl orthosilicate to form the polymer leads to the elimination of water molecules. In this condensation reaction, small irregular spheres of silica form, each a few angstroms in size. On reaching a particular size, these tiny particulates eventually aggregate with themselves during the reaction progress and form a large inorganic network polymer aggregate. The resultant porosity is determined from the size of the aggregating spheres and depends both on the reaction conditions and the process by which the gel is subsequently washed. During postreaction heating and drying, the gel shrinks, forming a rigid porous matrix. The dried silica gel is then ground and sieved to produce the desired particle size distribution. Microporosity can vary greatly but typical silica gels used in SPE provide a particle with a large surface area in the range of about 500–800 m2 /g. The entire particle, including the interior of the pores, is covered with free, highly polar silanol groups, which makes the particle available for polar analyte adsorption interactions or for covalent bonding reactions in the preparation of C18.
For SPE applications the nominal particle size is generally about 40–60 µm; however, the overall particle size will range in an approximate Gaussian distribution as shown in Figure 3. In some cases this may include particles as small as 2 µm and as large as 150 µm. Bimodal particle distributions (particle distributions having two maximum sizes) are also possible. Depending on quality control of the manufacturer attempting to maintain a consistent product, any significant shift in this overall size distribution can be especially significant when it comes to flow characteristics. A shift, for instance, to a smaller particle size increases flow resistance to liquids passing through a packed bed. This can be especially troublesome with liquids containing particulate matter, which can plug interstitial spaces.
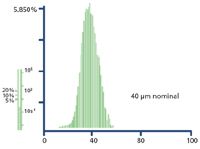
Figure 3: Typical particle size distribution (5).
In summary,
- Particle size affects flow characteristics, with larger particles providing better flow characteristics.
- Average particle size for most SPE columns is 40–60 µm.
- Narrow ranges of size distribution provide more consistent flow characteristics.
- Small particles result in extraction efficiencies similar to those of large particles.
Metallic Impurities
Another factor considered a variable in the adsorptive performance of a base silica material would be the presence of metallic impurities. These may take the form of alkaline and alkaline earth metals such as sodium, potassium, or calcium and magnesium. Other impurities that may be present include titanium and trivalent iron and aluminum (8), each forming high activity nonspecific adsorption sites. Impurities such as Al in the form of alumina contribute most characteristically to the behavior of the support, providing active hydroxyl sorption sites with high hydrolytic stability and polarity on the adsorbing surface. Although analysts have no control over the base quality of the silica gel used by a specific manufacturer, quality control methods by reputable manufacturers should ensure that the gel is consistent from lot-to-lot, thereby reducing variability. Silica manufacturers go to great lengths to synthesize gels with a minimum of metallic impurities to ensure dependable and reproducible analytical performance.
The Bonded Surface
Alkylsilane (C18) Formation
For silica gel to function as a nonpolar sorbent in SPE, some or all of the free hydroxyl groups must be converted to octadecyl (C18) functionality. This may be followed by an endcapping reaction depending upon what the manufacturer wishes to achieve for final product performance. Endcapping substitutes trimethyl groups for active silanol sites and renders them unavailable for polar sorptive interaction.
The primary bonding procedure may be performed in one of several ways. Bonded silicas are formed by the reaction of a monochlorodimethyl octadecyl silane with the active hydroxyl groups on the silica at a single point of attachment. This reaction liberates HCl and forms a silyl ether linkage — a stable covalent bond between the oxygen atom and the Si functional group along with the carbon functional group. Monomeric reactions form a one-layer coating of C18. Carbon loadings on such particles are generally limited to a maximum of about 12%.
Other configurations of bonding reactions are possible and are often used to make polymeric functionalized surfaces. Organosilanes such as dichloro- or trichlorosilanes have more than one reactive site, so multiple bonds in three dimensions can form. The resulting silica gel surface has greater hydrophobicity than monomeric forms and better stability in acidic conditions with pH values as low as pH 2–3. Carbon loadings of 25% and higher can be obtained using such silanes. The partial reaction of trichlorosilane is shown in Figure 4. The remaining chloride groups will also form silanol bonds, liberating a total of 3 mol HCl.
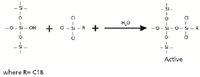
Figure 4: Trichlorosilane bonding to silica gel (6).
The purpose of the silane reaction is to cover the surface of the silica gel to provide properties that are primarily due to the chemical nature of the C18 carbon functional group. In real life, however, not all polar silanol groups are inactivated. C18 functional groups are bulky; steric hindrance from the bonded moiety can interfere with complete coverage of the silanol surface. This leaves residual hydroxyl groups on a silica surface, which makes the sorbent less hydrophobic than desired. With unbonded silanol functionality available for secondary polar interactions, a shift toward adsorption of polar analytes occurs.
Further issues may complicate surface coverage. Although various bonded gels may claim the same level of carbon loading, distribution of the carbon on the silica can vary significantly. Depending on the methods manufacturers use to conduct their reactions, many of which are proprietary, surface coverage may not be uniform. This is illustrated in Figure 5, which shows differing spatial arrangements of C18 functional groups that form a non-homogeneous surface. This is indicated in Figures 5a–5c. Carbon loading may be excessive in some regions and deficient in others, leaving large areas of unbonded free silanol. Ideally, surface coverage should exhibit good uniformity as shown in Figure 5d.
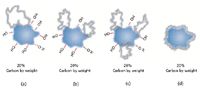
Figure 5: Carbon loading variations (5).
Another procedure introduces alkoxysilanes during the silica gel polymerization reaction. This process creates bulk modified silicas where the bonded phase forms on the surface and in the interior of the particle. Bulk polymerization reactions thereby create much more extensive coverage of the particle including the micropores. Better bonding coverage results in a higher overall carbon loaded particle with fewer exposed hydroxyl groups. With less polarity due to unbonded hydroxyl functionality of the phase, the loading capacity of the particle is increased for hydrophobic analytes.
In summary, the factors that limit bonding of silica include
- steric hindrance of bulky carbon groups that inhibit placement of adjacent functional groups
- intentional limits on the reaction coverage of the particle (stoichiometry)
- limits on the depth to which large bulky groups can penetrate deep pores in the gel
Endcapping
Limits to surface coverage from the primary functionalization reaction by bulky functional groups means that free hydroxyl groups will still be present. These hydroxyl groups present a surface that is available for adsorption of polar analytes. Although such polarity on a C18 surface is not necessarily an undesirable attribute, it affects the overall sorptive behavior of the solid phase toward hydrophobic analytes.
In an effort to minimize the extent of polar sites available for sorptive interaction, manufacturers inactivate free silanols by adding methyl groups in a process known as endcapping. Endcapping adds a small molecule such as trimethylchlorosilane (TMCS) to the silica gel after the primary bonding reaction is complete, further depolarizing the surface. HCl is liberated during this reaction. Depending on whether silica is endcapped or nonendcapped (it is commercially available in both forms), and the extent of functional group coverage, the ratio of bonded to unbonded surface will change, affecting the type and quantity of analyte that can be efficiently captured by the sorbent. Endcapping chemistry is shown in Figure 6.
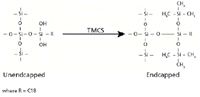
Figure 6: Silane endcapping reaction (TMCS = trimethylchlorosilane).
C18 Carbon Loadings
C18 products are available with a range of carbon loading. Table I shows sorbents available from several manufacturers. Overall carbon loading is indicated. Analysts may expect differences in loading efficiency and polarity of analytes captured among these brands.
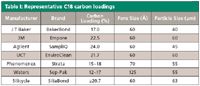
Table I: Representative C18 carbon loadings
Summary
As has been shown, C18 bonded silica gels can vary significantly. These differences are due to many factors, which include
- surface area of the silica gel particle
- metal impurities in the base silica gel
- particle size and distribution of the silica gel
- percent carbon loading of the silica gel surface
- type of carbon loading: polymeric or brush versus monomeric functionalization
- physical distribution of carbon pendent groups
- amount of unbonded or free silanol groups
- quality and extent of endcapping reaction (if endcapped)
- quality control in manufacturing.
Although this article is not meant to be comprehensive in all aspects of particle formation and bonding chemistry, it was written to introduce readers to the potential differences likely to be encountered in various C18 products currently on the market.
Craig A Perman is a chemist and the president of Aqua Technica, Inc. in Denver, Colorado. His consulting firm specializes in environmental water analysis and technical writing. He can be contacted by email at caperman7@gmail.com.
Michael Telepchak is with UCT, Inc. in Bristol, Pennsylvania.
References
(1) E.W. Abel et al, J. Chromatog. 22, 23–28, (1966).
(2) C.L. Zimmerman, T.J. Waeghe, and R.T. Moody, "Expanding the Capabilities of a C18 Bonded Phase by the Addition of a Pentafluorophenyl (PFP) Group," www.mac-mod.com/ppr/pdfs/C18-PFP%20poster%20HPLC%202010.pdf
(3) E.M. Thurman and M.S. Mills, Solid-Phase Extraction (John Wiley and Sons, Inc., New York, 1998).
(4) M. Telepchak, Forensic and Clinical Applications of Solid Phase Extraction (Humana Press, Totowa, NJ, 2004).
(5) M. Telepchak, "The Science of Solid-Phase Extraction," PowerPoint Presentation, http://www.edocfind.com/download/ppt/The%20Science%20of%20Solid%20Phase%20Extraction/aHR0cDovL3dlYi51Y29ubi5lZHUvcnVzbGluZy9UZWxlcGNoYWsucHB0 (2010).
(6) Library 4 Science, Chromatography-Online.org, http://www.chromatography-online.org/topics/spherical/silica.html
(7) Handbook of Thin Layer Chromatography, 3rd Ed Chromatrographic Science Series, J. Sherma and B. Fried, Eds. (Marcel Dekker, Inc., New York, New York, 2003).
(8) N. Simpson and K.C. Van Horne, Varian Sample Preparation Products, Varian Associates, Inc, (1993).
(9) N. Simpson, Solid-Phase Extraction, Varian Associates, Inc., Marcel Dekker, Inc., (2000).
(10) J. Nawrocki, Debbie L. Moir, and W. Szczepaniak, Chromatographia 28(3/4), 143 (1989).
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.








