The Case of the Too Big Little Peak
LCGC North America
A case of over-recovery of a preservative on ageing of a pharmaceutical product provides a good example of how to approach solving a problem of overlapping peaks in an LC separation.
What do you do when a peak grows over time?
I have been working with a woman who attended one of my recent classes to solve a problem with a liquid chromatography (LC) method in her laboratory. The problem makes a good case study for "LC Troubleshooting" because it allows us to review how to attack a problem that may not have an easy fix.
The method is for the analysis of a pharmaceutical product in a cream formulation. A preservative is added to the formulation at a 0.2% level, but has a puzzling behavior in one of the container types in a 12-month stability study at 25 °C. In a 1-oz tube, the preservative peak increases by about 20% from the day 1 concentration, but in a 2-oz tube under the same storage conditions, the same formulation shows no increase in the preservative. At a higher temperature, an accelerated stress condition, no change is seen in either container. The challenge is how to separate the preservative from the interference or identify the interference so that it can be eliminated, if necessary.
The method is run on a 150 mm × 4.6 mm, 5-µm particle C8 column thermostated to 35 °C and uses a 35:65 methanol–buffer mobile phase; UV detection is at 270 nm. The buffer is prepared by adding 1 mL of triethyl-amine (TEA) to 1 L of water and adjusting the pH to 3.0 with concentrated phosphoric acid. The sample is prepared by dissolving an aliquot in methanol, then diluting to 50:50 methanol–water followed by injection of 50 µL of this solution. The preservative peak is eluted at about 7 min with a flow rate of 1 mL/min.
When confronted with problems like this, I like to take a four-step approach:
- Determine what the goal is.
- Examine the existing method for potential problems.
- Plan some experiments that might solve the problem.
- See if there are any alternative approaches that make sense.
The goal of the current work is to isolate the impurity from the preservative. After this is done, a determination can be made to decide whether the formulation needs to be modified to avoid the appearance of the impurity or if it can be ignored. I suspect that it can be ignored, because at ~20% of a 0.2% peak, the concentration would be less than the 0.05% reporting level.
The Current Method
Let's next take a look at the existing method. Is there anything that raises a possible red flag? My first concern is that triethylamine phosphate (TEAP) is used as a buffer. This was a very common buffer when the older, low-purity, type-A silica columns were in use. In that situation, the addition of TEA to the mobile phase made sense, because it helped suppress active silanol groups on the silica-based column packing.
In those older columns, there was a significant amount of metal contamination, sometimes >100 ppm of iron and aluminum. These metals served to "activate" silanol groups to form cation-exchange sites on the surface. These were a primary cause of the significant peak tailing observed when bases were analyzed on such columns. Today, with higher-purity, type-B silicas used for the support in most columns, the metal content is reduced to <5 ppm in some cases, so the presence of ionized silanol groups is much less of a concern. This is especially true when the mobile phase pH is low, such as the pH 3 used in the present method. For these reasons, TEA is seldom used today. Does it hurt? I doubt it, but I'm a strong believer in the KISS principle: Keep It Simple, Stupid. In other words, extra additives to the mobile phase that don't contribute positively to the separation just make the mobile phase more complicated. And more complicated means more chances for something to go wrong. So this suggests to me that the method may have been one that was developed for use with columns from the past and just extended to new conditions.
A second concern with the current method is the injection of a fairly large volume of sample in a solvent that is stronger than the mobile phase. As a general rule, you don't want to inject more than ~15% of the volume of the peak of interest if you use the mobile phase as the injection solvent; less for a stronger solvent. To see if the current conditions are reasonable, we can make some estimates.
We need to estimate the peak volume of the peak of interest. This is based on the column efficiency and the retention time. A well-packed column operated with ideal test solutes will have a plate height, H, of ~2 particle diameters (dp); for more realistic conditions I like to use H ≈ 3dp. The column efficiency or plate number, N, can be calculated as

in which L is the length of the column (same units as H). If we assume H ≈ 3dp, we can estimate
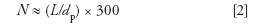
in which L is the column length in millimeters and dp is the particle size in micrometers. For the present column, then, N = (150/5) × 300 ≈ 9000. Let's call it N = 10,000 for more convenient mental math; we'll need the square root of this, 100, in a moment.
Now that we have an estimate of N, we need to figure out the peak volume. The standard way to calculate N from the chromatogram is

in which tR is the retention time and w is the peak width at baseline between tangents drawn to the sides of the peak. We can rearrange this and solve for w:

In our example, w = (4 × 7)/100 = 0.28 min. We need to convert this to volume, so 0.28 min × 1 mL/min = 0.28 mL = 280 µL. I mentioned above that for injection in mobile phase, it is good to inject no more than 15% of the peak volume. 280 µL × 15% = 42 µL. So the 50-µL injection would be a bit large even if it were in mobile phase, but the sample is in 50% methanol and the mobile phase is 35% methanol, so it is in a stronger solvent.
For stronger solvents, smaller sample volumes will be needed or extra peak broadening is likely. It would be easy to determine if this is a problem with the present method by injecting 50-, 25-, 10-, and 5-µL aliquots of sample and measuring the peak width or plate number of the first peak of interest.
If the smaller injections improve the plate number, the method should be changed accordingly. Perhaps it would be possible to dissolve the same amount of sample in a smaller volume of methanol and dilute with more water so that the sample concentration was the same, but the injection solvent was ≤35% methanol. Under such conditions, 50-µL injections would probably be fine.
Changing Selectivity
Our next task is to design some experiments that will allow us to separate the interference from the preservative. Complicating this is the presence of several other peaks in the chromatogram resulting from other formulation components and the active ingredient.
In several recent articles, we have looked at some of the variables that affect selectivity, especially the percentage of organic solvent in the mobile phase (1), the solvent type (2), and the column (3). In studies in our group (4), we have found that we can rank the separation power of selectivity variables for a wide range of compounds as
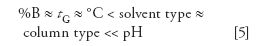
in which %B is the percent organic solvent in the mobile phase (10% change assumed), tG is the gradient time in gradient elution (threefold change), °C is the column temperature (10 °C change), solvent type is a change from methanol to acetonitrile or vice versa, column type is a change in column selectivity (5) (Fs) >65 (assumes ionizable compounds; >100 for nonionizable ones), and a pH change of 5 units (applies only to ionizable compounds).
I would start by investigating a ±10% change in methanol in the mobile phase and a ±10 °C change in the column temperature; gradient time does not apply to this isocratic separation. The Rule of Three suggests that retention will change about threefold for the 10% organic change. We would expect a change of ~15–20% in retention for a 10 °C temperature change if we assume a 1.5–2% change in retention for each 1 °C change in temperature. Rather than trying to identify the location of the interference peak, I would focus on the peak area of the preservative. With overlapping peaks, the peak area is about 120% of the expected area, so any reduction in this area is evidence that the separation is improving.
Next would be a change in solvent type, such as substituting acetonitrile for methanol. Because acetonitrile is a stronger solvent than methanol, I would start with 5–10% less acetonitrile, or 25–30% acetonitrile instead of 35% methanol. Again, watch for a change in the preservative peak area. Another option would be to use tetrahydrofran. Tetrahydrofuran can make large changes in selectivity, but is used less often today because of its poor UV transparency at <240 nm. However, the present method uses 270 nm for detection, so this limitation does not apply. It might be interesting to try tetrahydrofuran instead of methanol; I would start with 20–25% tetrahydrofuran. An alternative would be to substitute 10% of the methanol by tetrahydrofuran to obtain some selectivity difference.
When it comes to a change in the column type, consult the USP-PQRI database (3,5) to find a column with a significantly different selectivity. Because the interference is unknown, we don't know if it is ionizable or not, so I would look for a column that had a combined Fs value of >100 (listed in the database [5]) and a column B-value of >0.15.
I don't know if there will be changes in selectivity with a change in pH, because we don't know if the impurity is ionizable or not, but pH is a very powerful variable if ionizable compounds are present. When we are looking for significant changes in selectivity, a large change in pH is suggested; I would suggest 4–5 units of change.
In each case, when changing the variables, track the peak area of the preservative to look for changes. If two or more experimental runs are made with a variable (for example, two different temperatures), and changes in peak area are observed, it may be useful to use a retention mapping program such as DryLab (Molnar Institute, Berlin, Germany) to get more information from the collected data. If we use the reference conditions as one run and change only one variable (for example, the percentage of organic), we'll have the two calibration runs needed for retention mapping.
If none of the changes shows promise, you could change to a different chromatography mode. Normal-phase chromatography would be a good choice. Normal-phase chromatography separates compounds based on adsorptive interactions between the sample molecules and the stationary phase. Another option would be hydrophilic interaction chromatography (HILIC), which separates based on a combination of partition and electrostatic interactions. The separation mechanism of both of these techniques is significantly different from reversed-phase chromatography, which relies on hydrophobic interactions between the sample molecules and the stationary phase, so different selectivity would be expected.
An Alternative Approach
Another way to approach the current problem would be to try to isolate the impurity from the container. The impurity shows up when a smaller container size is used. One possible reason for this is that the surface area to volume ratio would be larger for the smaller container, so the impurity might be below a noticeable concentration in the larger tube. I would try to extract the impurity from the container, which is a plastic tube in the present case.
Start with a new tube without any labels on it. If a label-free tube is not available, try to scrape the inner surface to get sufficient sample without any printing on it. The tube or sample of the tube should be ground or chopped to increase the surface area, then extracted in organic solvent, such as by sonicating in a glass test tube. I would try extracting separate aliquots in a chlorinated solvent (for example, methylene chloride), methyl-tert-butyl ether, an aromatic solvent (for example, toluene), methanol, and acetonitrile. The objective is not to dissolve the tube, but to extract the impurity from the tube matrix. The extraction solvent then could be evaporated to dryness and the residue redissolved in a small volume of mobile phase.
Chromatograms resulting from injection of these extracts will contain, we hope, a peak that is eluted at the same retention time as the preservative. This peak presumably would be the interference. Now this sample could be used as a reference standard to be injected independently and compared with the retention of a reference standard of the preservative. When dealing with only two peaks and in independent injections, the effects of the investigations of the various selectivity variables above will be much easier to interpret.
If the isolated impurity can be separated from the preservative and the same conditions result in a reduction of the area of the preservative in the aged sample to ≤100% of the expected amount, it is likely that the isolated compound and the impurity are the same. A sample of the isolated material could then be submitted to mass spectrometry or other further analysis to determine its identity.
Conclusions
The present problem of over-recovery of a preservative on aging of a pharmaceutical product serves as a good example to explore possible approaches to solving a problem of overlapping peaks in an LC separation. The approach discussed above can be used as a general technique to solve similar problems in other sample types. It is important to establish the goals of the project before you start, then take a systematic approach to solving the problem. If you ignore these two important points, it is easy to get caught in a problem-solving approach that relies more on hope and luck than scientific discipline.
John W. Dolan"LC Troubleshooting" Editor John Dolan has been writing "LC Troubleshooting" for LCGC for more than 25 years. One of the industry's most respected professionals, John is currently the Vice President of and a principal instructor for LC Resources, Walnut Creek, California. He is also a member of LCGC's editorial advisory board. Direct correspondence about this column via e-mail to John.Dolan@LCResources.com

John W. Dolan
References
(1) J.W. Dolan, LCGC N. Am. 29, 28–34 (2011).
(2) J.W. Dolan, LCGC N. Am. 28, 1022–1027 (2010).
(3) J.W. Dolan, LCGC N. Am 29, 236–244 (2011).
(4) J. Pellett et al., J. Chromatogr. A 1101, 122–135 (2006).
(5) PQRI database athttp://www.usp.org/USPNF/columns.html.
A Novel LC–QTOF-MS DIA Method for Pesticide Quantification and Screening in Agricultural Waters
May 8th 2025Scientists from the University of Santiago de Compostela developed a liquid chromatography quadrupole time-of-flight mass spectrometry (LC–QTOF-MS) operated in data-independent acquisition (DIA) mode for pesticide quantification in agriculturally impacted waters.
Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









