Method Translation in Liquid Chromatography
LCGC North America
Converting a method from conventional HPLC to high-throughput HPLC or UHPLC is easy with a method translation calculator. Here's how to use them and where to find them, for free.
With the recent emphasis on high-throughput high performance liquid chromatography (HPLC) and ultrahigh-pressure liquid chromatography (UHPLC), chromatographers are looking for ways to improve productivity. This installment discusses the use of method translation calculators to make conversions from conventional HPLC to high-throughput HPLC and UHPLC more convenient to perform without the need for manual calculations. Both isocratic and gradient method translations are discussed and applications are demonstrated.
Nowadays everybody is interested in increasing productivity, and for good reason. Many analytical laboratories are experiencing increases in the number of samples with fewer personnel and the same number of instruments. Conventional gas chromatography (GC) and liquid chromatography (LC) methods were able to keep up with the sample load when only a few samples or tens of samples required analysis per day. Often, samples could be loaded into autosampler carousels and run overnight so that the next morning results could be provided to the requestor. However, many times those requesting the analytical data want (and perhaps need) the information more quickly than overnight.
Techniques for increasing the speed in chromatography laboratories have been known for years — shorter columns, faster flow rates, smaller internal diameter columns, and so on. The recent trends in high performance liquid chromatography (HPLC) column technology with shorter columns packed with smaller particles (1) or superficially porous particles (2) used at a higher flow rate are a direct result of the need for increasing sample throughput. In GC, shorter, smaller internal diameter columns perhaps with lower viscosity carrier gases, such as hydrogen, have resulted in faster separations (3). When higher throughput is required, analysts don't want to have to spend time redoing or revalidating the method but want to get the same elution order and resolution achieved with the original column but faster. To maintain the integrity of the separations, the experimental conditions must be adjusted so that everything looks the same, except that the analysis time is shorter.
The process of converting a method is termed method translation (also referred to as method migration, method conversion, or method porting). There are standard equations available to set up the new conditions and sometimes it is easy to calculate these parameters. For isocratic (HPLC) and isothermal (GC) work, the calculations are relatively straightforward. However, when one wants to translate gradient (HPLC) or temperature programming (GC), the calculations become a bit more challenging, especially if column internal diameters and lengths are also changed simultaneously. Fortunately, there are software packages available, mostly for free, to assist one in making a method change. Most of these free software packages have the name "Translator" or "Calculator" associated with their identification. The purpose of this installment is to demonstrate the use of method translation for some relatively simple HPLC isocratic and gradient methods and to provide sources where these calculators can be found. Because the equations forming the basis of these calculators can be found elsewhere, they won't be repeated here. I will provide some practical examples that can show one how to make a method conversion without having to perform manual calculations.
Even though GC method translation software was first developed in 1998 (3), I will cover HPLC method translation first because the calculations used in HPLC are simpler than in GC, mainly because the liquid mobile phases are relatively noncompressible compared to GC carrier gases. GC method translation will be covered in a future column installment.
Method Translation in HPLC
Here, I will consider isocratic method translation and then gradient method translation. Parameters that are used in isocratic HPLC method development using a single column type (for example, monomeric C18) and mobile phase combination that are considered in method translation include particle size, column length, column internal diameter, flow rate, retention factor, injection volume, sample concentration, and column back pressure. At this point in time, we will not include column temperature as a variable since variation in retention factor k with temperature can be compound-dependent. Because viscosity is influenced by column temperature, temperature is used in the method translation to calculate the mobile phase viscosity for water–acetonitrile and water–methanol mixtures. The pertinent equations used in HPLC method translation are well known and will not be covered here.
Isocratic Method Translation
To determine if an isocratic method is ready for method translation, one must set performance goals for the new method. The availability of the new column geometry to achieve the necessary efficiency must be established. The instrument needs versus the method performance goals must be assessed. One must determine if there are any instrumental restrictions, such as extracolumn effects, available data rate, and system pressure limitations. The flow rate availability from the solvent delivery system must be established as per the method performance goal for analysis time. If a smaller column volume is the outcome, the injection volume must be adjusted to compensate for it. If the injection volume is too small, one must assess injection repeatability and sample solvent composition robustness.
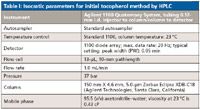
Table I: Isocratic parameters for initial tocopherol method by HPLC
For our first example, let's consider a simple isocratic separation of vitamin E α-, β-, and γ-tocopherols on a conventional 400-bar LC system using UV detection. The initial conditions and chromatograms of the reversed-phase separation are shown in Table I and Figure 1 (right-hand side), respectively. A 5-µm microparticulate column with the dimensions 150 mm × 4.6 mm was used with a 1-mL/min flow rate. Resolution of the three components was more than adequate for good quantitation. The separation time was just under 14 min, which in today's world is considered to be somewhat slow for such a simple analysis. A shorter column packed with sub-2-µm reversed-phase packing of the same type at a higher flow rate would provide a faster separation with equivalent efficiency. A goal of a factor of 5× in speed was set but using the existing equipment. In addition, saving solvent was a secondary goal for method translation. The question now arises about how much the method can be modified and which modifications are the most important.
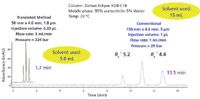
Figure 1: Comparison of conventional isocratic method vs. translated isocratic method at 3 mL/min.
Method Translator is free on-line software that allows users to first enter the parameters for an existing method and then specify parameters for a new method that provides an improvement (4). Through an automated system of "checks and balances" to ensure that one's system is capable of making a successful conversion, users then enter a new set of parameters. Figure 2 is a screen capture of the software tool modified for isocratic method translation. The left side of the screen provides the original LC method with some added parameter values. For example, the extracolumn dispersion of the standard LC system had a value of 35 µL derived from the volume of connecting tubing (21.6 µL because the tubing internal diameter was 0.17 mm) plus the volume of the UV flow cell (13 µL). The mobile phase was 95% acetonitrile–5% water, which had a viscosity of 0.43 cP. In this simple case, we wanted to adopt one of the newer, short sub-2-µm columns that should give similar performance to the original column but provide a faster separation.
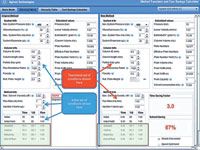
Figure 2: Screen capture: simple method conversion for isocratic separation of tocopherols.
The new method on the right side of Figure 2 shows that by use of a column one-third of the length, the time savings factor was 3.0 (constant flow of 1 mL/min) and the solvent savings was 67%, a substantial reduction in purchase and disposal costs. Because of the smaller column volume, a reduction in the injection volume to 0.33 µL was recommended. On the downside, because the original system was used without any further modification, the large system dispersion value (35 µL) gave rise to a loss of effective plates (down to 9849 plates from an expected 12,077). On such a short column, some adjustment of the extracolumn volume would have to be made to get a better representation of the potential efficiency gains. To achieve a reduction in extracolumn volume, the original connecting tubing was replaced with smaller 0.12-mm i.d. tubing and the 13-µL flow cell was replaced with a 5-µL semimicro flow cell, cutting down the extracolumn volume to 11 µL. By reducing the extracolumn volume by roughly two-thirds, the effective plates now numbered 11,300 and the resolution was unaffected. Still, our goal of a factor of five in speed was not achieved. A simple click on the "Speed Optimized" button on the Method Translator page showed a recommended increase in the flow rate to 3 mL/min as shown in Figure 3 (the right-hand side of the screen capture only). By making these method modifications recommended by the Method Translator program, we have decreased the separation time to 1.7 min (a factor of 8.9 over the original method) and have ended up saving nearly 10 mL of total solvent per run. Table II summarizes the new set of conditions. The chromatogram on the left hand side of Figure 1 shows that the peaks were still well resolved with good peak shape. The pressure did increase to 224 bar but this was within the pressure capability of our system so it presented no problem.

Figure 3: New speed-optimized translated isocratic method for tocopherols.
This example shows how using a method translation package, one can optimize an isocratic method without having to perform innumerable calculations. Many of the performance parameters are automatically adjusted as one enters the new column and instrument configuration. The calculations also showed the importance of minimizing the extracolumn effects on efficiency when using sub-2-µm ultrahigh-pressure liquid chromatography (UHPLC) columns.
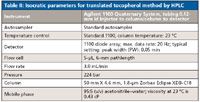
Table II: Isocratic parameters for translated tocopherol method by HPLC
Gradient Method Translation
The use of gradient elution is the most popular approach to analyzing complex samples, reducing analysis time, increasing peak capacity, and increasing sensitivity. One can also use method translation to convert gradient methods to improve overall performance. When one considers converting a gradient method, equation 1 is the relationship that makes it easier to understand the tradeoffs in modifying the chromatographic conditions:
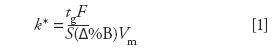
where
k* = apparent retention factor under gradient conditions; note that the actual retention factor changes constantly during a gradient;
Δ%B = difference between the initial and final %B values; multistep gradients and those with gradient holds in the middle of the gradient profile are more difficult to translate;
S = constant (~4–5 for molecules for small molecules (100–500 Da) and 10–1000 for large biomolecules such as peptides and proteins);
F = flow rate (mL/min);
tg = gradient time (min); and
Vm = column void volume (mL).
In gradient method translation, one desires to keep the relative peak positions in the chromatogram unchanged and as long as equation 1 is satisfied, a method should be translated easily. Any change in one parameter on the right-hand side of the equation 1 should be offset by a proportional adjustment of another parameter. Table III outlines some simple changes that have to be made in order to balance equation 1.
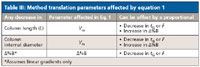
Table III: Method translation parameters affected by equation 1
As with isocratic method translation, in gradient method translation performance goals must be established by first examining the current method. In addition to the performance parameters discussed in isocratic method translation, parameters such as column volume (Vm, affected by column length and internal diameter), the gradient program (hopefully linear), dwell volume (also called gradient delay volume — the point of solvent mixing in the flow system to the head of the column), resolution of critical pairs, and column back pressure (mobile phase viscosity can change dramatically during gradient elution) become more important and should be considered in gradient elution. Think of any tradeoffs that you may have to make in the new method: pressure or flow limitations of your LC system, columns on hand (different phases, dimensions, hardware configurations), extracolumn effects (some of which may be negated by the focusing of analytes at the head of the column), instrument gradient delay volumes (that may change with pressure due to presence of pulse dampers), post-gradient equilibration (regeneration) time, time constant of detector, or data rate of your data system.

Figure 4: (a) Original method using conventional HPLC. (b) Gradient method translation using simple conversion mode. (c) Gradient method translation using speed-optimized mode.
Let's now consider the method translation of a gradient example. Using the same Method Translator online program as for the isocratic approach, we desired to replace an older method for the analysis of impurities of an active pharmaceutical ingredient (API) done by conventional HPLC. Figure 4a shows the conventional separation of the API and its impurities on a 250 mm × 4.6 mm column packed with 5-µm StableBond C18 reversed-phase particles (Agilent Technologies). The analysis was done under typical conditions often used in pharmaceutical QA/QC laboratories. At a temperature of 40 °C, the entire separation required about 20 min using a 5–90% gradient over 20 min with a 3-min isocratic hold and a 7-min regeneration step. It was desired to convert the method to a sub-2-µm column (100 mm × 4.6 mm) to maintain the resolution of the impurities present. Using the "Simple Conversion" mode (screen capture not shown here), the overall separation time was decreased to a little more than 7 min (a time savings factor of 2.5) with a 60% savings in solvent (42 mL in original method to 16.8 mL in translated method). The new chromatogram is depicted in Figure 4b. Method Translator calculated all the setup parameters including the new gradient times and injection volume. The resolution was maintained but the pressure at the maximum solvent viscosity of the water–acetonitrile mixture (0.75 cP at 40 °C obtained from a viscosity table on another tab on the same Method Translator website) increased from 95 to 292 bar as a result of the smaller particles packed in the column. Finally, clicking on the "Speed Optimized" button on the Method Translator program, the flow rate was increased from 1.4 mL/min to 2.58 mL/min, taking into account the maximum pressure rating of the instrument. Figure 4c provides the highest speed separation with the separation time just under 4 min, a time savings factor of 4.6 compared with the original conventional LC method. As expected, the operating pressure increased to 540 bar, which is still below the 600-bar capability of the HPLC system that was used for this assay. A screen capture of the speed-optimized gradient translated method showing both initial and final conditions is depicted in Figure 5. A further calculation using the "Resolution Optimized" mode could have been performed but the overall analysis time would have been longer and more solvent would have been used.
Figure 5: Speed-optimized gradient method translation for API and its impurities.
If the method required the use of mass spectrometry (MS) detection where such high flow rates are not MS friendly, then a 100 mm × 2.1 mm column could have been entered into the translated method, resulting in the use of only 3.5 mL of solvent per run (not shown here), because of the lower flow rates used to maintain the proper linear velocity with this smaller internal diameter column.
Although the number of displayed parameters in the Advanced Method Translation tool may look formidable, many of them are based on calculated values. Method Translator starts with a preset of values for every input field. There is also a "Basic Mode" of operation where certain values required for calculations (for example, flow resistance and porosity) that are not commonly known are preset and hidden from view to simplify the interface. All calculated values are updated immediately after any input value is changed. All inputs are checked for consistency: For example, is the flow rate adequate for the column being used as well as the solvent and temperature? Are the gradient entries logical?
The Method Translator software tool can be used for any commercial liquid chromatograph where some of the input parameter values must be entered manually. A convenient "Cost Savings Calculator" is available through another tab on the Method Translator website to help to determine total savings for adopting shorter, faster analytical columns.
Where Can You Get Method Translation Programs?
There are a number of method translation programs or free services for HPLC that are available from both commercial and academic sources. As mentioned earlier, they are almost all "freeware" and can be used online or downloaded onto your PC. Table IV lists the locations for some of these programs.
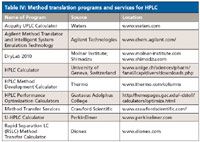
Table IV: Method translation programs and services for HPLC
Conclusion
In this installment, I have demonstrated the use of method translation software to make conversions from HPLC to high-throughput HPLC and UHPLC more convenient to carry out without the need for laborious manual calculations. Both isocratic and gradient method translations were discussed and applications demonstrated. Most of the software packages are free and can be used online or downloaded. Sources for some of these programs have been provided.
Ronald E. Majors "Column Watch" Editor Ronald E. Majors is Senior Scientist, Columns and Supplies Division, Agilent Technologies, Wilmington, Delaware, and is a member of LCGC's editorial advisory board. Direct correspondence about this column via e-mail to lcgcedit@lcgcmag.com.

Ronald E. Majors
References
(1) R.E. Majors, LCGC No. America 26(1), 16–28 (2008).
(2) R.E. Majors, LCGC No. America 28(12), 1014–1020 (2010).
(3) L.M. Blumberg and M.S.Klee, Anal. Chem. 70, 3828–3839 (1998).
(4) http://www.chem.agilent.com/en-US/Products/Instruments/lc/Pages/1200infinity_cost_calculator.aspx
Perspectives in Hydrophobic Interaction Temperature- Responsive Liquid Chromatography (TRLC)
TRLC can obtain separations similar to those of reversed-phase LC while using only water as the mobile phase.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)





