What’s New in the Analysis of Complex Environmental Matrices?
The Column
The Royal Society of Chemistry’s Environmental Chemistry Group, Water Science Forum, and the Separation Science Group Joint Meeting was held on Friday 3 March 2017 at the Science Suite of the Royal Society of Chemistry, in Burlington House, Piccadilly, London, UK.
Photo Credit: Sergej Razvodovskij/Shutterstock.com

Roger Reeve1 and Graham Mills2,1Sunderland Pharmacy School, University of Sunderland, Sunderland, UK, 2School of Pharmacy and Biomedical Sciences, University of Portsmouth, Portsmouth, UK
The Royal Society of Chemistry’s Environmental Chemistry Group, Water Science Forum, and the Separation Science Group Joint Meeting was held on Friday 3 March 2017 at the Science Suite of the Royal Society of Chemistry, in Burlington House, Piccadilly, London, UK.
The meeting on 3 March 2017 at the Royal Society of Chemistry (RSC), Burlington House, London, UK, was organized by the Environmental Chemistry Group, the Separation Science, and the Water Science Forum. The monitoring and screeningâtype analysis of a wide range of both regulatory and emerging pollutants (such as pharmaceuticals and personal care products) was a major theme along with the detection of potentially hazardous compounds in dusts and consumer articles. There was also an exhibition by instrument manufacturers and suppliers of laboratory consumables.
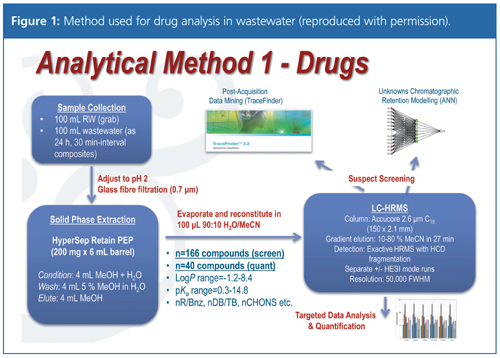
The first presentation, “Screening of Complex Forensic and Environmental Samples Using High Resolution Analysis and In-Silico Data Mining Tools”, was given by Leon Barron (King’s College London, UK). Analysis of contaminants in water can be divided into targeted analysis of known compounds, untargeted analysis indicating all resolvable components, and, between these, suspect screening. In-silico methods can help with identification of chemicals in suspect screening. Applications were given of pharmaceuticals and personal care products, illicit drugs, and explosives. The analytical method used for drugs is shown in Figure 1. Artificial neural network analysis of the components can predict chromatographic retention times and was used for the preliminary identification of new compounds. Log D, log P, number of C/O groups, and benzene rings contributed most to the fitting. This analysis complemented identification by data mining of the high-resolution mass spectroscopy data. The methods were not meant to replace the traditional use of standards, but to optimize the analytical effort needed for such a large number of components.
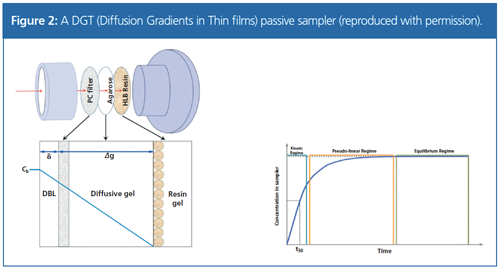
Andrew Sweetman (Lancaster University, UK) discussed the “Use of Passive Samplers as a Potential Compliance Tool Within the EU Water Framework Directive”. These samplers can give time-weighted average concentrations as an alternative to averaging a time series of grab water samples. The sampler investigated was DGT (Diffusion Gradients in Thin films), as shown in Figure 2, originally developed for metals but also now used for antibiotics, personal care products, pharmaceuticals, pesticides, and flame retardants. The sampler is left to sequester pollutants over 1–2 weeks, after which time the accumulated components are extracted from the sampler resin and the masses determined. Using known diffusion rates, this can be related back to the freely dissolved concentration. Dissolved concentration reflects the biologically available fraction, but does not directly provide the “total concentrations” required by the EU Water Framework Directive. An estimate of this value can be made using partition theory.
Colin Creaser (Loughborough University, UK), in his presentation “Combining Ion Mobility Spectroscopy with Mass Spectroscopy for the Analysis of Complex Samples: The Potential for Environmental Analysis”, discussed two formats of ionâmobility spectroscopy (IMS). In driftâtube ion mobility spectroscopy (DTIMS), separation is by the differential mobilities of ions in the presence of an electric gradient and a buffer gas. Field asymmetric ion mobility spectroscopy (FAIMES) has an oscillating field that selects individual ions-similar to quadrupole mass filters in conventional mass spectrometers-and can be used at atmospheric pressure. This technique is transportable and can be used in field-based applications. It is easy to use, has rapid response, and high sensitivity, but has a narrow dynamic range (1–2 orders of magnitude) and low resolution, which leads to its use in applications involving simple mixtures, such as BTEX (benzene, toluene, and xylenes). To overcome the limited resolution within the laboratory, the technique can be coupled to conventional mass spectrometers and has been applied to the targeted analysis of sulfonylurea herbicides and haloacetic acids in water. For nontargeted analysis, collisional crossâsections, derived from the drift time, can be used for analyte identification. This was illustrated by identification of cyclophosphamides in wastewater. There is improved performance over standard methods for targeted high throughput quantitative analysis and increased peak capacity for nontargeted applications.
Stuart Harrad (University of Birmingham, UK) discussed the problem of “Brominated Flame Retardants in Waste Consumer Articles”, such as found in carpet underlays and furniture, building insulation foam, and electronic waste (e-waste). E-waste must be separated from other waste streams and material recovery is tightly controlled. In less developed countries e-waste disposal may be rudimentary and the compounds may enter the wider environment and hence the food chain. Leaching from furniture and waste fabrics is easy and highlights the need for more sustainable waste management for these products. The enormity of the problem was confirmed by a detailed analysis of brominated flame retardant concentrations in a wide range of consumer products.
The first keynote lecture “Nontarget Screening of Environmental Pollutants in the Context of Risk Assessment of European River Basins: The NORMAN Network Perspective” was presented by Jaroslav Slobodnik (Environmental Institute, Kos, Slovak Republic). The NORMAN network within the EU has been set up to enhance the exchange of information on emerging environmental substances, their validation, and harmonization of analytical techniques. There is currently no good explanation of the ecological status of most European river basins and current priority substances do not explain all the effects. What impact have emerging pollutants (pharmaceuticals, personal care products, biocides, transformation products) had? Little attention is given to metabolites and transformation products or the effect of mixtures. Of the many compounds present, only a few determine the risk, hence the need for prioritization. The NORMAN network and ECOTOX database provide the methodology and resources for this prioritization.

Jacob de Boer (Vrije Universiteit, Amsterdam, Netherlands) discussed “Human Exposure to Environmental Contaminants: Direct Probe Time-of-Flight Mass Spectroscopy Reveals a Multitude of Chemicals Indoors”. The talk began with brominated flame retardant compounds and then followed with nonbrominated alternatives including chloroparafins and phosphorus-based compounds, which are found in indoor dust. Direct probe mass spectroscopy, which introduces the solid sample directly into the ionization chamber of the mass spectrometer, has been used as a fast screening technique for compliance with legislation, typically 5 min for each sample. The method avoids losses found in techniques using separate extraction and separation.
Monitoring taste and odour substances in drinking water is a regulatory requirement linked to acceptable “wholesomeness” of potable water. Gavin Mills (Severn Trent Water plc, UK) described “Advances in the Identification of Tastes and Odours in Drinking Water” including the use of instrument top sample preparation (ITSP) cartridges, which provide solidâphase extraction cleanup of the sample as part of the automation system. Gas chromatography triple quadrupole mass spectrometry (GC–TQMS) is useful for routine work whereas gas chromatography quadrupole time of flight (GC–QTOF), with greater sensitivity and selectivity, allows simultaneous determination of targeted and nontargeted compounds. A supplementary “detector”, known as an odour port, allows individually eluting compounds to be matched to the odours.
Erika Castringanò and Luigi Lopardo from Barbara Kasprzyk-Hordern’s group (University of Bath, UK) asked the question “Can Urban Water Profiling Inform our Understanding of the State of Environmental and Public Health?” Medicinal drugs are often misused as drugs of abuse and can appear in wastewater. The drugs are chiral and when used legitimately are a single enantiomer. Drugs bought on the street contain a mixture of enantiomers. Analysis using chiral liquid chromatography (LC) columns allows an estimation of the relative proportions of the legal and illicit use. Investigations of water profiling have been extended to endocrine disruptors and determination of metabolic biomarkers. In vitro studies combined with urban water analysis can be used to determine public exposure.
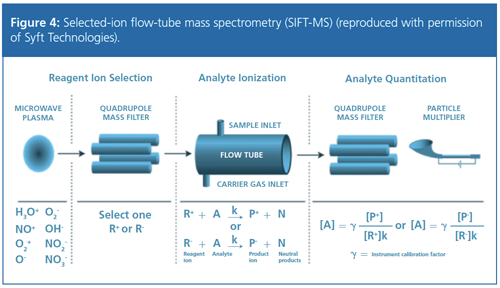
Mark Perkins (Anatune Ltd., UK) described “VOC Measurements in Ambient Air Using Selected Ion Flow Tube Mass Spectrometry – Automation and Calibration Considerations” to produce an autonomous air monitoring system. The selected-ion flow-tube mass spectrometer (SIFT-MS) technique, as shown in Figure 4, involves the chemical ionization of the analyte by selected positive precursor ions produced from humidified air. No external gas supplies are needed, which allows autonomous field use. Current field trials are evaluating the robustness and reproducibility of the technique in routine field deployment, and determining the accuracy and precision for determining realâtime trace-level volatile organic compound (VOC) mixing ratios.
The meeting was the third in the biennial series on the analysis of complex environmental matrices and its success leads to the event likely to be repeated in early 2019.
The industry meeting sponsors were Agilent Technologies, Anatune Ltd., Hichrom Ltd., Imspex Diagnostics Ltd., Kinesis, Markes International, and Thames Restek Ltd. Financial support is also acknowledged from Environment Sustainably and Energy Division of the RSC.
Roger Reeve lectures in inorganic and analytical chemistry in Sunderland Pharmacy School, University of Sunderland, Sunderland, UK.
Graham Mills is a professor of environmental analytical chemistry in the School of Pharmacy and Biomedical Sciences, University of Portsmouth, Portsmouth, UK.
E-mail: roger.reeve@sunderland.ac.uk / graham.mills@port.ac.ukWebsite:www.rsc.org/membership/networking/interestgroups/environmental/index.asp

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)