Use of Electron Capture Negative Ion Mass Spectra to Establish the Identities of Polybrominated Diphenyl Ether Flame Retardants and Their Degradation Products
Special Issues
Detrimental health effects of a group of brominated flame retardants, polybrominated diphenyl ethers (PBDEs), have been recognized recently, but only after their wide usage and consequently, global dispersal. Of the possible 209 PBDE congeners, 39 (varying in degree of bromination from mono to deca) have been identified previously in the three common technical mixtures. Additional congeners, presumably debromination products of the fully brominated decabromodiphenyl ether (BDE-209), also have been reported in biotic and abiotic environments. However, costly analytical standards are needed to confirm their identification. In addition, the most widely used identification approach, electron ionization (EI) mass spectrometry (MS), primarily produces spectra indicating only homologue grouping (for example, hepta-BDE). Without specific compound identification, full assessment of toxicological consequences of PBDE burdens is impeded. It has been reported previously that electron-capture negative ionization (ECNI),..
Polybrominated diphenyl ethers (PBDEs) are a class of brominated flame-retardant (BFR) additives, common in plastics (for example, electronic casings, computers, and televisions), circuit boards, polyurethane foam (furniture padding), and textiles. These widely used chemicals have been manufactured since the mid-1970s. However, following evidence that constituents of these mixtures bioaccumulate and disrupt biological processes such as the endocrine system, the manufacture of two of the three common formulations (penta- and octa-BDEs) was discontinued in the U.S. in 2004. This followed their ban by the European Union earlier that year (2). These two formulations, as well as the still-manufactured deca- (consisting of >97% by weight of BDE-209), are considered environmentally persistent and globally dispersed (3,4). Sources include industries producing or using PBDEs, as well as releases from finished products (5,6). The latter suggests that PBDEs will continue to be released into the environment long after the 2004 cessation in production. Even before termination of penta and octa production, deca- was the most widely used PBDE formulation [>83% 2001 global production (7)]. It is considered a lesser health concern because of its lower bioaccumulation potential, attributed to its extreme hydrophobicity and significant molecular mass (959 Da). However, reports have indicated that BDE-209 is bioavailable (8–10), although to a lower extent than the constituent congeners of the penta- and octa-formulations. Continued use of deca-BDE might be problematic because it has been reported to undergo abiotic [photolytic (11) and metal oxide reduction (12)] and biotic debromination (9,10), thus potentially adding to existing burdens of the lower brominated diphenyl ethers.
Like polychlorinated biphenyls (PCBs), 209 different PBDE congeners are feasible, and the same IUPAC scheme is used for their naming. In reality, the three commercial mixtures tend to be much simpler than those of PCBs, consisting primarily of 39 individual PBDE congeners (7). This is due to the directing influence of the oxygen and the large size of the bromine atoms (Figure 1). PBDEs are analyzed routinely by gas chromatography (GC) coupled with electron-capture detection (ECD), or more commonly, low resolution mass spectrometry (LRMS), operated either in electron ionization (EI) or electron- capture negative ionization (ECNI) modes. High resolution MS (HRMS) also has been used and has a number of advantages over LRMS (such as increased sensitivity and selectivity), but it requires more experienced users and is much more costly and labor intensive (13). Selective ion monitoring (SIM) ECNI (e.g., monitoring bromine ions ([79Br]– and [81Br]–) has been shown to be a very sensitive technique and is used widely for the determination of low sub-parts-per-billion (ppb) levels. However, like ECD, this technique can result in misidentification (for example, 2,294,49,5,69-hexabromodiphenyl ether (BDE-154) is coeluted with 2,294,49,5,59-hexabromobiphenyl (BB-153) (14). Further spectral information can be obtained by scanning multiple ions, or an extended range of ions, at the loss of some sensitivity.
Ion fragment clusters centered on the molecular ion [M]+ and the loss of two bromines [M-2Br] have been observed previously in PBDE EI spectra (13). This approach, in combination with retention-time data, has found utility for compound identification. However, acquisition of standards for all 209 PBDEs can cost from $20,000 to $30,000, and most searchable mass spectra libraries presently do not contain PBDEs (for example, NIST/EPA/NIH Mass Spectral Library with Search Program: NIST 05, Ver. 2.0d). So most laboratories focus on a subset of congeners previously identified in the three commercial mixtures. "Unknowns" typically are only characterized to the level of homologue (for example, hepta-BDE). Consequently, this lack of specificity limits specific congener identification, toxicological studies, and degradation pathways for these quasi-labeled PBDEs.
However, scanning the full range of ions in ECNI provides additional PBDE structural information, often permitting compound identification. For example, constituents of the penta-formulation were identified tentatively using GC–ECNI-MS spectra as penta- and hexa-BDEs by observing ion fragments representing the molecular ion [M]– and fragments indicating the loss of sequential bromine ions ([M-Br]–, [M-2Br]–, and [M-3Br]–) (1). Similar fragments also were observed for a tetra-BDE in the mixture, but the molecular ion was not present. Stemmler and Hites (1) also reported that increasing the ion source temperature from 100 °C to 250 °C resulted in the production of an additional ion cluster in the hexa-BDE spectra, indicative of cleavage at the ether bond. Unlike EI, where molecules are ionized directly through interaction with high-energy electrons, in ECNI, an analyte interacts with a lower energy reagent gas, such as methane or isobutene. The reagent gas molecules, at about 1 torr pressure in the source, are excited by an electron beam (~200 eV). Analytes with an affinity for electrons (such as halogenated compounds) will form negative ions, which are stabilized by the higher pressures produced by the reagent gas. This mode often is referred to as soft ionization; ECNI imparts significantly less energy than EI, resulting in less fragmentation. Also, at lower internal energy, fragmentation or ion abundance is influenced greatly by the ion source conditions (temperature and pressure) (15). These parameters can then be modified, producing structural information in ECNI mode complementary to that of EI alone. As stated previously, EI spectra of PBDEs generally exhibit the molecular ion [M]+ and fragments representing the loss of two bromines [M-2Br]+ . However, increasing the ECNI ion source temperature from 100 °C to 250 °C results in cleavage of the ether bond, producing ion clusters centered around 328 and 330 m/z, indicative of a benzene ring containing three bromines ([C6Br3H2O]–) (1). Additional GC–ECNI-MS studies also have observed PBDE cleaving with ions centered on 408 m/z, 486 m/z, and 488 m/z, diagnostic of a benzene ring containing four ([C6Br4HO]–) and five ([C6Br5O]–) bromines, respectively (7,16). Unlike EI spectra, which only permit classification to the level of homologue group, the ECNI spectra can vary as a function of bromine position on the phenyl rings. Consequently, analysts can elucidate the structure of some congeners using this technique, or at a minimum, eliminate some possibilities.
Experimental
Individual PBDE standards (Table I) were analyzed by GC (6890N, Agilent Technologies, Palo Alto, California) with MS detection (JMS-GC Mate II, JEOL, Peabody, Massachusetts) using ECNI and methane (99.99%) as the reagent gas. Samples were introduced (1-μL) into the split–splitless injector, equipped with a 1-mm i.d. glass liner, using a 15-m retention column (J&W Scientific, Agilent Technologies, DB-5HT, 15 m × 0.25 mm, 0.1-μm stationary phase) to separate analytes from their carrier solvent (hexane). The injector temperature was 300 °C, and initial carrier gas (helium) head pressure was 50 psi. This injection "pressure pulse split–splitless" technique has been reported to be suitable (minimum BDE-209 thermal degradation) for mono- through deca-PBDE analysis (7,14,17). The split vent was opened and pressure was reduced to 15.2 psi (flow 1.5 mL/min) after 4 min, following sample injection. Thereafter, column flow rate was kept constant throughout the remaining portion of the run by increasing carrier gas pressure to the head of the column, compensating for increasing oven temperatures, following the ideal gas law. Initial column oven temperature was 90 °C, held for 4 min, and then increased to 150 °C at 30 °C/min, then 10 °C/min to 300 °C, and held for 7 min. It was then increased to 350 °C at 30 °C/min and held at 350 °C for 5 min, as a bake-out procedure.
The mass spectrometer was tuned and calibrated using a 1000:1 mixture of perfluorokerosene (PFK) and 1,2,4-trichlorobenzene, using ion 331 m/z, resolution 1200. Mass calibration included 84 points, ranging from 35.0 to 998 m/z. Typical values for the ion source were: electron energy 200 eV, filament 300 μA. A source temperature of 200 °C was observed to be optimal to produce fragmentation characteristic of PBDE ether cleavage and molecular ions for the higher brominated compounds. ECNI mass spectra were collected in full scan (10 to 1000 m/z), at 1.0 scans/s. All reported spectra were identified at greater than one-half peak height. In addition to ionization mode, sample concentration can influence fragmentation patterns observed in the spectra (for example, percent abundance of minor ions). Therefore, for spectra to be relevant to environmental contaminant levels, PBDE standard concentrations used in this study were approximately 500 pg on-column.
Results and Discussion
The most abundant ion observed in 59 spectra of the 64 PBDE standards, analyzed by GC–ECNI-MS, was the bromine ion [Br]–, 79 and 81 m/z. Spectra for the other five PBDEs contained major fragments corresponding to cleavage of the ether bond. These corresponded to 409 m/z ([C6Br4HO]–) for BDE-179, -197, -201, -202, and ions 487 and 489 m/z ([C6Br4HO]–) for BDE-209. Each spectrum was normalized to the bromine ion signal and % abundance calculated for each of the other major fragment ions detected (Table I). The spectra for all 64 PBDEs can be viewed at www.vims.edu/env/people/staff/laguardia.html. As mentioned previously, ECNI is a softer ionization technique than EI, generating PBDE spectra corresponding to [C6BrxHyO]–, cleaving at the ether bond. In this study, similar patterns also were observed primarily for PBDEs with greater than six bromines. The molecular ion [M]– followed by fragments derived from the loss of bromines ([M-Br]–, [M-2Br . . . ]–) also was detected, mostly for PBDEs containing greater than five bromines. It also was observed that bromine positioning (ortho, meta, and para) on the diphenyl rings influenced fragmentation patterns greatly. These observations indicate that acquisition of ECNI spectra is more powerful for PBDE identification than EI alone.
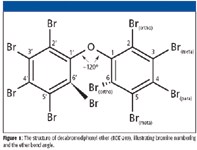
Figure 1
Unlike PCBs, in which nonorthosubstituted rings can rotate more freely, PBDE rotation is hindered by the oxygen bond angle (~120°) between the rings (Figure 1). This influences bromine placement and compound stability. When subjected to ECNI, opposing bromines in the ortho (6,69) position (Figure 1) contribute stress to the oxygen bond, causing the PBDE to be cleaved at the ether bond. This is indicated by an intense fragment corresponding to [C6BrxHyO]–, as observed in each PBDE spectrum containing bromines in the ortho (6,69) position (that is, BDE-155, -179, -184, -188, -197, -201, -202, and -204) (Table I). It also was apparent that in ECNI, bromines in the para (4,49) positions have a stabilizing effect on the ortho-position bromines, allowing for molecular ion identification. For example, the molecular ion centered at 802 m/z was detected for BDE-197 (Figure 2a); however, the molecular ion ([M]–) was not observed for BDE-201 (Figure 2b). The difference was due to a shift of the para (49 position) bromine in BDE-197 to the meta (59 position) in BDE-201 (indicated by the arrows in Figures 2a and 2b).
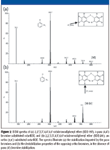
Figure 2
Cleavage at the ether bond also produces spectra indicating the number of bromines per phenyl ring (Table I, columns [C6Br3H2O]–, [C6Br4HO]–, and [C6Br5O]–). Hepta-BDEs with five bromines on one ring and two on the other (referred to as a [5,2] bromine distribution) did not produce spectra indicating cleavage at the ether bond (Figure 3a). This might be an indication of the stability provided by the fully brominated phenyl ring or the lack of opposing bromines in the ortho (6,69) position. However, the [4,3] distributed hepta-BDEs did produce ion clusters at 329, 331, and 409 m/z ([C6Br3H2O]– and [C6Br4HO]–, respectively), indicating cleavage at the ether bond (Figure 3b). However, three [4,3] hepta-BDEs (BDE-171, -183, and -191) did not produce this type of signal. These contain stabilizing para (4,49) position bromines and lack destabilizing opposing bromines at the ortho (6,69) position. Octa-BDEs also displayed a signal indicating phenyl ring bromine distribution, either [4,4] for BDE-194, -196, -197, -201, and –202 or [5,3] for BDE-198, -203, and -204. One octa-, BDE-205, did not demonstrate this, but again, this might be due to nonopposing ortho bromines and fully substituted para bromines (as seen in BDE-171, -183, and -191). The three nona-BDEs had strong signals indicating [5,4] bromine distribution, and the strongest ion cluster observed in the deca-BDE (BDE-209) spectra was produced by cleavage at the ether bond, [C6Br5O]– (Table I).
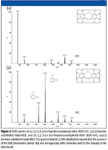
Figure 3
By interpreting ECNI spectra in the manner described earlier, it was possible to support the identification of previously suspected metabolic debromination products of BDE-209, the primary component of the deca-formulation. These PBDEs were apparent in biota collected downstream from the outfall of a wastewater treatment plant, which received BDE-209 from a plastic manufacturing facility (10). One octa-BDE could not be identified conclusively by EI. However, examination of the ECNI spectra revealed ether cleavage, a strong signal at 409 m/z with no observed molecular ion. This indicates an even distribution [4,4] of bromine on each phenyl ring, ortho (6,69) substitution, and no para (4,49) substitution. Of the 12 possible octa-BDEs, 7 are [4,4] bromine distributed and only one (BDE-202) does not contain para substituted bromines. This indicates that BDE-202 is a likely match for the unknown. Two unknown hepta-BDEs also were identified in the same manner. Their spectra indicated [4,3] bromine distributions, characteristic of strong ion signals centered on 409 m/z [C6Br4HO]– and 329, 331 m/z [C6Br3H2O]– . However, one spectrum did contain a slight signal for the molecular ion. Of the possible 24 hepta-BDEs, 18 are [4,3] distributed, but only 4 have opposing bromines on the (6,69) ortho position (BDE-176, -179, -184, and -188), needed to produce cleavage at the ether bond. BDE-184 was eliminated as a possibility because of the stabilization properties of its bromines in the (4,49) para positions. This produces a strong molecular ion signal, which was absent in the unknown spectrum. However, BDE-176 and -188 only contain single bromine in the para position; thus, these remain as possible candidates. The other peak was identified as BDE-179, because it does not produce a molecular ion signal, characteristic of the lack of para (4,49) bromines.
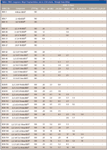
Table I: PBDE congeners: Major fragmentation ions in ECNI (mono- through hexa-BDEs)
Conclusion
Application of ECNI, a softer ionization technique than EI, can produce spectral information that is invaluable for the identification of PBDE congeners. Historically, interpretation of ECNI-generated data has been utilized rarely due to variations in instrumental conditions and configurations, thereby creating nonreproducible spectra. However, as instrumentation has evolved, parameter monitoring and control have improved greatly. This allows the analyst better control of ionization processes, reducing inconsistencies between spectra. These technological improvements permitted the exploitation of this alternative ionization technique, resulting in the generation of a ECNI spectra library for 64 PBDEs. Mass spectral interpretation permitted the confirmation of the identities of a number of BDE-209 metabolic debromination products.
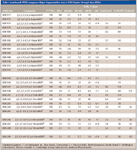
Table I (continued): PBDE congeners: Major fragmentation ions in ECNI (hepta- through deca-BDEs)
Acknowledgments
The author would like to thank Dr. Robert C. Hale and Ellen Harvey for their assistance in manuscript preparation. This paper is contribution number 2922 from the Virginia Institute of Marine Science, The College of William and Mary.
Mark J. La Guardia is with the Department of Environmental & Aquatic Animal Health, Virginia Institute of Marine Science, The College of William & Mary, Gloucester Point, Virginia; e-mail address: markl@vims.edu
References
(1) E.A. Stemmler and R.A. Hites, Electron Capture Negative Ion Mass Spectra of Environmental Contaminants and Related Compounds (VCH Publishers, Inc., New York, 1988), pp. 278–280.
(2) R. Renner, Environ. Sci. Technol. 38, 14a (2004).
(3) Y. Su, H. Hung, E. Sverko, and H. Li, Atmos. Environ. 41, 8725–8735 (2007).
(4) Y. Wang, G. Jiang, S.K.P. Lam, and A. Li, Environ. Int. 33, 963–973 (2007).
(5) S. Hazrati and S. Harrad, Environ. Sci. Technol. 40, 7584–7589 (2006).
(6) R.C. Hale, S.L. Kim, E. Harvey, M.J. La Guardia, T.M. Mainor, E.O. Bush, and E.M. Jacobs, Environ. Sci. Technol. 42, 1452–1457 (2008).
(7) M.J. La Guardia, R.C. Hale, and E. Harvey, Environ. Sci. Technol. 40, 6247–6254 (2006).
(8) K. Jakobsson, K. Thuresson, L. Rylander, A. Sjödin, L. Hagmar, and Å. Bergman, Chemosphere 46, 709–716 (2002).
(9) H.M. Stapleton, B. Brazil, D. Holbrook, C.L. Mitchelmore, R. Benedict, A. Konstantinov, and D. Potter, Environ. Sci. Technol. 40, 4653–4658 (2006).
(10) M.J. La Guardia, R.C. Hale, and E. Harvey, Environ. Sci. Technol. 41, 6663–6670 (2007).
(11) M-Y. Ahn, T.R. Filly, C.T. Jafvert, L. Nies, I. Hua, and J. Bezares-Cruz, Environ. Sci. Technol. 40, 215–220 (2006).
(12) M-Y. Ahn, T.R. Filly, C.T. Jafvert, L. Nies, and I. Hua, Chemosphere 64, 1801–1807 (2006).
(13) A. Covaci, S. Voorspoels, and J. de Boer, Environ. Int. 29, 735–756 (2003).
(14) P. Korytár, A. Covaci, J. de Boer, A. Gelbin, and U. Brinkman, J. Chromatogr., A 1065, 239–249 (2005).
(15) E.A. Stemmler and R. Hites, Biomed. Environ. Mass Spectrom. 15, 659–667 (1988).
(16) J. Björklund, P. Tollbäck, and C. Östman, J. Mass Spectrom. 38, 394–400 (2003).
(17) J. Björklund, P. Tollbäck, C. Hiärne, E. Dyremark, and C. Östman, J. Chromatogr., A 1041, 201–210 (2004).

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Quantifying Terpenes in Hydrodistilled Cannabis sativa Essential Oil with GC-MS
April 21st 2025A recent study conducted at the University of Georgia, (Athens, Georgia) presented a validated method for quantifying 18 terpenes in Cannabis sativa essential oil, extracted via hydrodistillation. The method, utilizing gas chromatography–mass spectrometry (GC–MS) with selected ion monitoring (SIM), includes using internal standards (n-tridecane and octadecane) for accurate analysis, with key validation parameters—such as specificity, accuracy, precision, and detection limits—thoroughly assessed. LCGC International spoke to Noelle Joy of the University of Georgia, corresponding author of this paper discussing the method, about its creation and benefits it offers the analytical community.











