A Comparison of ICP-OES and ICP-MS for the Determination of Metals in Food
Special Issues
The determination of inorganic elements in food substances is critical for assessing nutritional composition and identifying food contamination sources. The inorganic elements of interest can be divided into two classes: nutritional and toxic. It is important to determine the levels of both sets of elements accurately to assess both the nutritional and the harmful impacts of food substances. Nutritional elements such as Mg, P, and Fe are present at high levels (milligrams per kilogram), while toxic elements such as Pb, Hg, and Cd should be present only at trace levels (nanograms or micrograms per kilogram).
Inorganic analysis of food for metals that might be harmful to health is an important part of ensuring food safety. Metals can contaminate raw products through exposure to contaminated soil or water during growth. Contamination during transport is also possible. During processing, gross or trace contamination can be introduced through machinery used. Product packaging can contribute to the contamination and was the subject of a U.S. FDA import alert describing lollipop candies contaminated with lead from the packaging ink. Levels of metals permissible in food are not defined clearly in U.S. regulations, although some guidance has been developed through prior encounters with contaminated food (1).
This work compares techniques for metal measurement in a variety of foods using inductively coupled plasma-optical emission spectroscopy (ICP-OES) and inductively coupled plasma-mass spectrometry (ICP-MS). The suitability of the techniques for analytical capability and productivity will be compared.
Experimental
A variety of food samples were purchased and digested for analysis. Microwave-assisted digestion (Mutiwave 3000, PerkinElmer, Shelton, Connecticut) was used following the program in Table I, taking approximately 0.5 g of sample in replicate and using 6 mL of nitric acid and 1 mL of hydrochloric acid (acids were Optima grade and were obtained from Fisher Scientific, Pittsburgh, Pennsylvania).
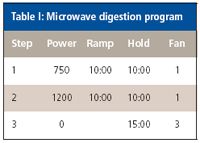
Table I: Microwave digestion program
The samples were analyzed using ICP-OES and ICP-MS using the parameters listed in Table II and Table III.

Table II: ICP-OES conditions
Results and Discussion
The samples were chosen to represent a variety of matrices for analysis. The U.S. FDA import alerts were used for guidance on what types of food samples had shown unacceptable levels of toxic metals when tested for import. Although similar matrices were chosen, predigestion spikes were used to demonstrate an elevated level of metals, because the samples were not expected to be contaminated. Table IV shows some of the items listed in import recalls, the country of origin, the contaminating element, and the levels observed in testing.

Table III: ICP-MS conditions
Samples purchased from a local grocery store included the following foods and beverages:
Orange soda drink — 1 (Mexico)
Orange soda drink — 2 (Canada)
Grape juice (origin not stated)
Guanabara nectar — canned (Mexico)
Raisins (California)
Apricots (Turkey)
Lollipops (Mexico)
Lollipop inner wrappers (Mexico)
Because the U.S. does not have regulations specifying toxic elements and prescribed levels permitted in foods, elements generally considered toxic were examined, including Sb, As, Cd, Cr, Hg, Pb, and Se. Se is considered an essential nutrient in various forms, but it can be toxic at higher concentrations. Other elements such as Mo and Sn also might be of interest. The samples were measured with ICP-OES, and the results are shown in Table V.
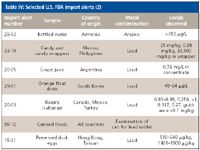
Table IV: Selected U.S. FDA import alerts (2)
The samples were spiked with 0.5 mg/L of each element of interest except Hg before digestion.
Results show very little contamination measured, although recoveries of the spikes were very good. Due to the dilution during digestion, this would be a good technique for screening for high levels of contamination. If nutritional elements were being measured, generally at milligram-per-kilogram levels, ICP-OES can be used easily for potentially toxic elements for a screening technique. Table VI compares the estimated detection limits in solution for ICP-OES and ICP-MS. ICP-MS detection limits are lower and will be important in measuring lower levels of contamination without digesting larger quantities of food.
The wavelengths used for ICP-OES measurement are shown in the first column with the element symbol. The masses used for ICP-MS measurement are listed in the third column, except for Pb, which is actually the sum of masses 206, 207, and 208. The results for the same samples run with ICP-MS are shown in Table VII.
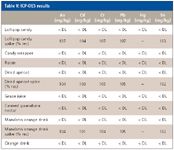
Table V: ICP-OES results
In this case, the results for each digestion replicate are shown, as well as the results for the postdigestion spike of 1 ng/L for As, Cd, Cr, Pb, and Se, and 0.1 ng/L for Hg. In the case of the orange drink sample, lead is present at a level that still might be of concern, especially for small children who might drink fruit-flavored drinks often. This level was below that which could be observed reliably with ICP-OES with the amount of sample digested here.
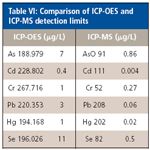
Table VI: Comparison of ICP-OES and ICP-MS detection limits
To ensure that the developed digestion and measurement procedures are under control, a reference material, NIST 1548a Typical Diet, was carried through the same procedures. Table VIII shows the results for the elements of interest.
The recovery of the certified values was very good, indicating that ICP-MS coupled with the digestion procedure can measure lower concentrations in the original solid material accurately.
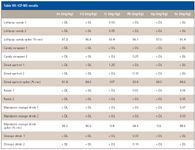
Table VII: ICP-MS results
Food matrices can be complicated, and interferences at the mass of interest can occur from molecules arising from the plasma gas or samples. These challenges can be overcome using cell-based ICP-MS, which has the ability to measure both high and low levels while removing the effects of interferences. Arsenic was measured using the dynamic reaction cell (DRC) to compensate for any interferences that might be encountered from interferences such as 35 Cl40 Ar on the only isotope of arsenic (mass 75) and to provide the lowest detection limit. Cell-based ICP-MS systems work by introducing a gas into a cell located in the path of the ion beam emitted from the ICP. Interferences and analytes interact differently with the gas, resulting in reduction of the interfering species. As a result, low levels of analytes can be detected and measured, even in the presence of interfering species.
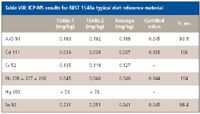
Table VIII: ICP-MS results for NIST 1548a typical diet reference material
Conclusion
A variety of food matrices were digested and measured using ICP-OES and ICP-MS. ICP-OES is useful for measuring higher concentrations, such as would be seen with nutritional elements or high levels of contamination. When lower levels of contamination are present, ICP-MS will provide lower detection limits for measurement. In addition, cell-based ICP-MS provides an additional tool for removal of interferences that might prevent detection of a contamination incident.
Zoe Grosser, Kenneth Neubauer, Laura Thompson, and Lee Davidowski are with PerkinElmer Analytical Sciences, Shelton, Connecticut.
References
(1) Candy Guidance, www.cfsan.fda.gov/~dms/pbcandy2.html (2006).
(2) Import Alerts available at www.fda.gov/ora/fiars .

Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.








