Understanding and Improving Solid-Phase Extraction
LCGC Europe
Pages 645-652
In this instalment of "Sample Prep Perspectives", we cover some of the basic scientific principles behind solid-phase extraction (SPE) to allow the correct mode of extraction to be selected through an understanding of how analytes interact with and are separated by the sorbent. We also look at some of the most common causes of low recovery in SPE and how they can be investigated and remedied. Finally, we examine some of the emerging technologies within the area.
Sample preparation accounts for up to 60% of the time taken for the analytical process. Therefore, it is important to select the correct sample preparation technique and understand the underlying separation mechanisms to fully maximize the process to give optimum results. Solid-phase extraction (SPE) is a highly selective mode of sample preparation, akin to the familiar principles of column chromatography. The range of currently available SPE sorbent chemistries and new technologies makes this technique applicable to a wide variety of analytes in myriad application areas. However, when compared to other sample preparation techniques such as protein precipitation, liquid–liquid extraction (LLE), or QuEChERS (quick, easy, cheap, effective, rugged, and safe), SPE can be considerably more time consuming and often requires a much greater effort towards method development. However, the rewards are certainly worth it, with highly selective extractions typically producing very pure samples.
In this instalment, we cover some of the basic scientific principles behind SPE to allow the correct mode of extraction to be selected through an understanding of how analytes interact with and are separated by the sorbent. We also look at some of the most common causes of low recovery in SPE and how these can be investigated and remedied. Finally, we examine some of the emerging technologies within the area.
SPE Mechanisms
SPE sorbent phases fall into four primary categories: nonpolar, polar, ion exchange (cation and anion), and mixed mode.
Nonpolar SPE: Nonpolar SPE phases contain nonpolar functional groups such as C18, C8, C6, C4, C2, phenyl, cyclohexyl, and cyanopropyl. Interaction between the analyte and sorbent surface is done via van der Waals (sometimes called dispersive) forces. These sorbent types are used for the extraction of molecules that contain nonpolar functional groups from predominantly polar matrices (for example, water). The interaction between the analyte and the sorbent surface group is facilitated by polar solvents, which repel the analyte from the solution phase and more strongly onto the sorbent surface. To elute analytes from any sorbent surface, the interactions between the analyte and SPE functional groups must be disrupted. In the case of nonpolar surfaces, this can be achieved through the use of solvents with some nonpolar character (that is, less polar than water, such as methanol, acetonitrile, isopropanol, or tetrahydrofuran). As mentioned previously, this extraction mode is highly amenable to the extraction of nonpolar analytes from polar matrices and is applicable in areas such as the analysis of pharmaceuticals, environmental samples, and clinical samples (1,2).
Polar SPE: Polar SPE phases are used for the extraction of polar analytes from nonpolar matrices. The sorbent surface has polar functional groups such as diol, aminopropyl, cyanopropyl, unbonded silica, alumina, and Florisil (US Silica). Analytes containing polar functional groups can interact and, therefore, be retained at the sorbent surface via dipole–dipole or hydrogen bonding interactions. To maximize analyte-sorbent interaction, nonpolar solvents should be used, and to disrupt these interactions to elute the analyte, a solvent with some polar character should be used (for example, tetrahydrofuran, ethyl acetate, isopropanol, acetonitrile, methanol, water, and combinations of water-miscible organics with water). If the polar analyte is already in a polar medium such as a biological sample (which would not allow good retention on the sorbent surface), a simple solvent exchange (via liquid–liquid extraction) into a nonpolar solvent can be performed before the SPE extraction.
Ion Exchange: Ion-exchange media come in both anionic and cationic forms for the extraction of analytes with basic (cationic) or acidic (anionic) functional groups. Cation-exchange sorbents contain surface groups that are negatively charged and the reverse is true for anion-exchange materials. Both surface types interact with the oppositely charged analyte via electrostatic (ionic) interactions. There are three mechanisms for disrupting analyte or sorbent interactions:
- Using a high ionic strength buffer. The large number of ions within the buffer will disrupt the analyte or sorbent interactions and elute the analyte.
- Altering the pH through the addition of an acid or base. This mechanism can work in two ways. The pH can be adjusted so that the analyte is neutralized and eluted from the SPE sorbent or the functional groups on the surface can be neutralized for a similar effect. As a general rule in deciding whether to raise or lower the pH, to neutralize an acidic functional group, lower the pH two units below the pKa (of the analyte or surface functional group). Conversely, for basic functional groups, increasing the pH two units above the pKa (of the analyte or surface functional group) will deprotonate the functional group, rendering it neutral.
- Utilizing buffers that contain counterions that have a high affinity for the sorbent surface. If the analyte is strongly retained by the surface and the interaction cannot be disrupted by using a high ionic strength buffer. These high-affinity ions can readily displace the analyte, which may have a lower affinity for the sorbent surface. This effect is dramatically more pronounced in anion exchange than in cation exchange. The relative selectivity of different counterions is shown below.
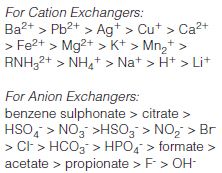
To change to a more highly selective ion, pass 2–5 bed volumes of 1 N solution of the new counterion through the sorbent. To change to an ion with lower selectivity, pass 5–65 bed volumes (depending on how much less selective the new counterion is in comparison to the current counterion) of 1 N solution of the new counterion through the sorbent.
Ion-exchange sorbents can be further classified as either weak or strong exchangers depending on the type of ionic group bonded to the surface. Strong cation exchangers contain an acid functional group such as a sulphonic acid, which is ionized over the entire pH range. Weak cation exchange sorbents have surface functional groups such as carboxylic acids that are negatively charged at high pH, but neutral at low pH. Similarly, strong anion exchangers include quaternary ammonium groups that are ionized (positively charged) over the full pH range. Weak anion exchangers have primary, secondary, or tertiary amine moieties that are ionized at low pH, but neutral at high pH. The flow diagram shown in Figure 1 can be used to select an appropriate ion-exchange sorbent.
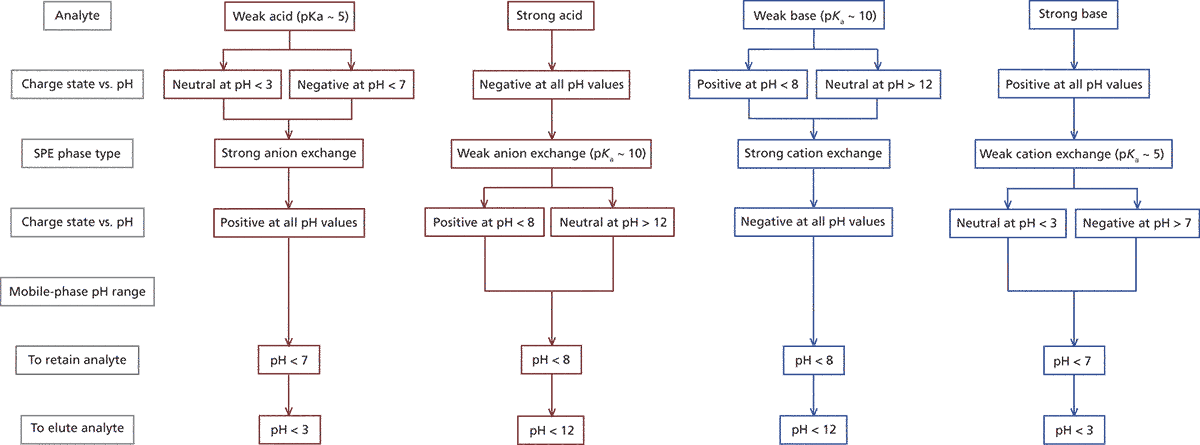
Figure 1: Ion-exchange selection chart (including analyte and sorbent properties).
For both anion and cation exchange, a protic environment is required for ion exchange to occur (water is highly protic). Some biological samples like urine or certain cell-growth buffers exhibit very high salt content (thus, high ionic strength) and may require dilution to reduce the ionic strength before application to the SPE sorbent to facilitate retention.
Mixed Mode: Mixed-mode sorbents exhibit two or more primary retention mechanisms with the most common mixed-mode sorbents having hydrophobic and ion-exchange functional groups attached to the surface. Hydrophobic groups can vary from short chain, less retentive (but more selective) C2 moieties to highly retentive C18 groups. The ion-exchange functionalities can be anion exchangers, cation exchangers, or a combination of both. There are two methods of manufacturing mixed-mode sorbents, either by bonding the sorbent concurrently with different functional group chemistries or by blending discrete sorbents in the appropriate ratios. The blending approach is preferable because of the ease of bonding a single functional group to the silica surface reproducibly. Furthermore, if different retentive properties are required, different ratios of single functional group sorbents can be blended. The development of protocols using mixed-mode sorbents can be more involved than with sorbents that have a single retention mechanism; however, very clean extracts from highly complex matrices can be achieved.
To elute analytes from the surface of a mixed-mode sorbent, which retains the analytes via two retention mechanisms, both retentive interactions must be disrupted. With sorbents that have both hydrophobic and ion-exchange groups, this will often involve the use of mixtures of nonpolar solvents with either appropriate buffers, acid, or bases.
Sorbent Selection
There are three questions that should be asked prior to choosing an SPE sorbent:
- What is the target analyte?
- What is the sample matrix?
- How much sample is to be analyzed?
The choice of SPE sorbent is determined by the analyte functional groups and polarity. To understand what interactions need to be optimized to facilitate separation of the analyte from any interferences, the properties that are unique to the analyte should be considered. These include ionic properties (pKa), presence of aromatic rings, and polarity. For example, a molecule that is very hydrophobic is ideal for separation on a reversed-phase sorbent. Analytes with acidic or basic functional groups will be well retained on ion-exchange media.
The sample matrix also needs to be taken into consideration when selecting an SPE sorbent. For example, aqueous samples should be separated using reversed-phase sorbents. Samples in organic solvents are suitable for normal-phase sorbents. Occasionally, some sample pretreatment may be required to place the sample in the correct matrix for the desired separation mechanism. For example, the use of polar SPE sorbents with biological samples may require a solvent exchange into a nonpolar solvent. If the sample is in the solid state, the solubility will need to be determined to allow homogenization in either an organic or aqueous solvent. Most SPE manufacturers will have flow charts that can be used to aid in the selection process (Figure 2).
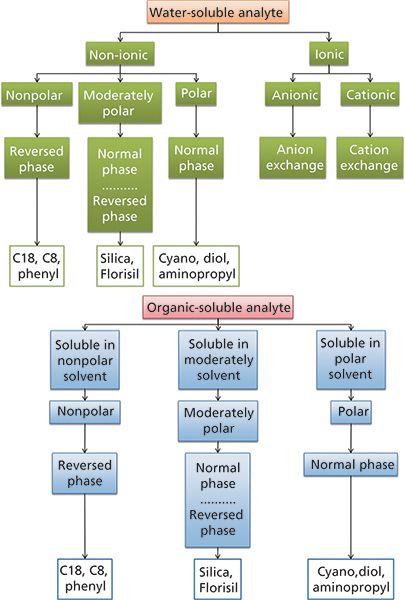
Figure 2: Examples of flow charts for SPE sorbent selection.
Finally, after the appropriate sorbent type has been selected, the sorbent mass of the SPE cartridge needs to be chosen. The choice of sorbent mass is dependent on the sample concentration and the analytical technique being used. The sorbent capacity refers to the mass of retained compounds (analytes and other components) that may be held by a given mass of the sorbent. There are some simple rules of thumb that can be used to determine the sorbent mass:
- Silica-based sorbents: The mass of retained solute is 5% w/w of the sorbent mass; that is, a 100-mg cartridge can retain 5 mg of total solute.
- Polymeric sorbents: Since polymeric sorbents have a larger surface area in comparison to silica-based sorbents, they have a larger capacity, approximately 10–15% w/w of the sorbent mass.
- Ion-exchange sorbents: For ion exchange sorbents, the capacity is determined by the number of available charged sites and can range from 0.8 to 1.3 meq/g. The minimum sorbent mass for ion-exchange sorbents can be calculated using equation 1:
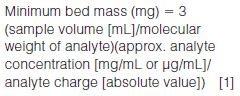
Protocol Steps in SPE
Each of the protocol steps must be optimized during method development. Not all of the protocol steps will be discussed in detail in this article. However, an in-depth optimization of sorbent equilibration, drying, and soak steps is discussed here.
Sample Pretreatment: Pretreatment is required to make the sample compatible with the SPE procedure and to optimize the sample chemistry to promote analyte retention. This often involves three primary steps: particulate removal (filtration); sample chemistry adjustment to facilitate retention (pH adjustment, dilution); and freeing bound analytes (analytes that are bound to other sample components, that is, particulates or proteins).
Conditioning: Conditioning the SPE sorbent removes any impurities that might remain from the manufacturing process and activates the sorbent surface to promote analyte interaction. For hydrophobic phases, conditioning is carried out by a water-miscible organic solvent (methanol, acetonitrile, or tetrahydrofuran). If the sorbent is water-wettable or polar, then conditioning may not be needed but is still recommended.
Equilibration: Equilibration is performed just before loading the sample. The function of equilibration is to create a sorbent chemistry environment that is similar to that of the sample. This step is required so that application of the sample does not change the chemistry of the SPE sorbent, which could lead to irreproducible results and poor analyte recovery. The pH is often an important parameter that needs to be considered during equilibration. For example, if a sample is pretreated to have a pH of 3.5 and the column is equilibrated at a different pH, during the course of sample loading the pH of the sorbent environment will be altered. This pH difference will result in the initial portion of the sample experiencing a different extraction environment to that experienced by the latter portion. This can affect retention characteristics, result in analyte breakthrough, and ultimately cause irreproducible results and poor analyte recovery.
Furthermore, it is important to ensure that any equilibration solvents are miscible with the sample to avoid precipitation of analyte or sample components. Precipitation may lead to analytes not being properly retained or an increase in back pressures resulting in poor processing of the SPE cartridge. Again, ultimately analyte precipitation will result in poor reproducibility and low analyte recoveries.
Sample Loading: The most important parameter of this step is the linear velocity of the sample as it passes through the column. Linear velocity is a function of both flow rate and column. One unique feature of SPE is that the sorbent can be chosen to selectively retain sample components and contaminants, while the analyte of interest is not retained. Ultimately, this means that during sample loading the analyte is not retained on the sorbent, but simply passes through with the loading solvent as a clean extract. This sorbent selection for sample retention may not work with all sample types, but if it does it can considerably shorten the sample preparation time.
Column Washing: Washing selectively removes undesired contaminant species. The solvent used for washing steps has a higher elution strength than the sample solvent, but is weaker than the elution solvent to ensure that analytes are not eluted, which would lead to low recovery.
Analyte Elution: The elution solvent must be capable of disrupting all of the retentive interactions between the analyte and sorbent, but not too strong to remove tightly bound contaminants.
Additional SPE Steps
There are two steps that are not included in the generic SPE protocol, but can be used to a great advantage. Therefore, they are worth understanding and considering during SPE method development. They are the soak and drying steps.
Soak Step: During a soak step the solvent applied to the column is allowed to remain within the cartridge, allowing time for chemical and physicochemical equilibria to be established between the solvent and sorbent. A soak step can be used during sorbent conditioning to improve sorbent surface activation and is often used with nonpolar sorbents, especially phases that have been endcapped. Using a soak step during sorbent conditioning can improve analyte retention and reduce the effects of variable flow rates through the cartridge. Typically, for a 100-mg sorbent mass a soak step of 30 s to 2 min would be used.
Soak steps can also be used during analyte elution. During elution, the solvent can be allowed to remain in the cartridge, allowing time for the analyte or sorbent interactions to be fully disrupted and for the analyte to diffuse into the elution solvent. This approach can be advantageous because it allows the number of solvent aliquots used to perform analyte elution to be reduced (this in turn reduces the final extract volume, which can reduce subsequent dry down times). Furthermore, ensuring that analyte sorbent interactions are completely disrupted allows for maximum analyte recovery. Finally, any flow-rate dependence of the elution step will be mitigated.
Drying Step: A drying step may be included after the wash steps. Drying steps can be particularly important with aqueous samples when elution is carried out using a water-immiscible solvent, because water that remains at the surface may exclude the elution solvent, leading to poor recoveries. Additionally, water in the eluate may increase drying times. Polar sorbents generally require longer drying times because of their water wettability. Drying steps may be reduced or completely eliminated when water-miscible solvents are used for analyte elution. Normal drying times range from 5 to 20 min.
Recovery Problems
The most common causes of low recovery during SPE are analyte breakthrough during sample loading, elution of analytes during wash steps, and the use of elution solvents that are not strong enough to fully elute the analyte of interest. To assess any problems with recovery it is important to define the overall recovery of the extraction process. Recovery of the extraction procedure (RE) is given by equation 2 (3).

where ResponseExtracted sample is the average area count for the analyte in the matrix that has been through the extraction (SPE ) process and ResponsePost-extracted spiked sample is the average area count for the same quantity of analyte spiked into extracted matrix after the extraction procedure.
Furthermore, when techniques such as liquid chromatography–mass spectrometry (LC–MS) are being used, the matrix can cause suppression or enhancement of the analyte signal. The matrix effect (ME) can be calculated from equation 3 (1).

If a recovery problem is discovered, first verify that the problem is not associated with a change in the chromatographic system or detector. This can be done by injecting known concentrations of standards in the final SPE extraction solvent to verify the response. Absolute recoveries of both target analytes and internal standards should be calculated and compared with injections of neat standards.
If the problem is not with the chromatographic system, each step of the SPE protocol must be examined to assess where the analytes are being lost. This can be done by processing analyte standards in pure solvents through the extraction procedure and collecting fractions from each of the protocol steps. These fractions should then be analyzed for the presence of analytes.
After the step associated with the loss has been identified, the cause of the low recovery needs to be identified and remedied. For example, if there is analyte breakthrough during the loading step, it should be verified that the correct solvents are being used in this step and prior steps such as conditioning and equilibration. If the solvent turns out to be incorrect, the method may need to be redeveloped with alternative solvents that can enhance retention. If the solvents are not the cause of the loss of analytes and breakthrough is a new phenomenon, then the SPE sorbent should be questioned. A comparison of different sorbent lots with the original sorbent should highlight if the sorbent is the problem. If wash steps are identified as being problematic, the same procedures detailed above can be used to re-optimize the SPE protocol and remedy any recovery issues.
If the analytes are strongly retained and not eluted from the SPE sorbent, there are several parameters that should be assessed. Again, verifying that the correct solvent is being used and that there has been no change in the sorbent chemistry are good starting points. Following this step, the elution strength of the solvent may need to be increased to facilitate quantitative elution. It is also possible that there could be secondary interactions between the sorbent and analyte that preclude proper elution; for example, interaction between basic analytes and surface silanol groups may predominate. Finally, if recovery is still poor it may be necessary to change to a less retentive sorbent.
If all protocol steps seem to be working correctly, it may be that the analyte is not reaching the column at all. At this point any preloading steps (protein precipitation, analyte stability, and so on) should be assessed.
Modern SPE Sorbents
SPE is still an advancing area with novel sorbent chemistries being developed to meet the needs of chemists, such as polymeric sorbents, molecularly imprinted polymers, and restricted-access media. Let's look at those sorbents in more detail.
Polymeric Sorbents: Polymeric sorbents are comprised of organic polymers. Therefore, there is no silica support and no surface silanol groups that can contribute to unwanted secondary interactions. Polymeric sorbents have a much higher surface area, hence, they have a higher loading capacity compared to traditional silica-based sorbents. Silica is inherently unstable at extreme pH (at low pH the surface groups will be hydrolyzed from the sorbent surface, whereas at high pH the silica itself is at risk of hydrolysis). Polymeric sorbents, however, have a much larger pH stability range (pH 0–14), allowing them to be used for applications that require these extreme separation conditions. A variety of chemistries (including analyte specific phases) have been developed to separate various analyte types. One final advantage of polymeric sorbents is that they do not have the flow-rate dependence of more-traditional silica-based materials.
Molecularly Imprinted Polymers: Molecularly imprinted polymer sorbents consist of highly cross-linked polymers that have a predetermined selectivity for a single analyte or group of structurally related analytes. The selectivity is introduced during the synthesis of the sorbent by using a template molecule to form specific cavities. Because of the high selectivity of these sorbents, they provide lower detection limits. With LC–MS, a reduction in ion suppression will be apparent because of the removal of matrix components. Again, because they are polymeric phases, the pH and temperature stability exhibited by these types of phases is often greater in comparison to silica-based media.
Restricted-Access Media: Restricted-access media (RAM) phases are highly specialized sorbents that combine size exclusion with another retention mechanism. For example, internal surface reversed-phase (ISRP) RAM are composed of silica whose inner pores (~120 Å) are functionalized with reversed-phase moieties (C18, C8, C4) while the outer surface is hydrophilic and consists of a material such as methyl cellulose. Large molecules that cannot access the pores are not retained, whereas smaller molecules are retained. These types of sorbents are particularly useful for the removal of matrix components (proteins, nucleic acids) from complex biological samples such as blood, serum, urine, and milk. They have also been used in on-line sample preparation applications and have exhibited higher recoveries than off-line SPE (3,4).
In the past, polymeric, molecularly imprinted polymers, and restricted access media sorbents were restricted to use in academic and research settings; however, they are now becoming more widely available through commercial suppliers. These advances are indicative of the continued growth of SPE into a routine technique in a variety of analytical laboratories.
Dawn Watson, PhD, is the technical expert for CHROMacademy, which allows her to use her interests in science communication and the development of on-line scientific training. Dawn has extensive experience in wet chemistry techniques and the use of a wide array of analytical techniques for reaction characterization and monitoring including HPLC, LC–MS, IR, NMR, and GC techniques. Her commercial experience was gained through the sales and support of automated instrumentation for HPLC, SPE, and LC–MS applications. Her academic research focus was in synthetic inorganic chemistry.
Douglas Raynie is an Associate Research Professor at South Dakota State University, USA. His research interests include green chemistry, alternative solvents, sample preparation, high resolution chromatography, and bioprocessing in supercritical fluids. He earned his PhD in 1990 at Brigham Young University under the direction of Milton L. Lee.
"Sample Prep Perspectives" Editor Ronald E. Majors is an analytical consultant and is a member of LCGC Europe's editorial advisory board. Direct correspondence about this column should be addressed to "Sample Prep Perspectives", LCGC Europe, Honeycomb West, Chester Business Park, Wrexham Road, Chester, CH4 9QH, UK, or e-mail the editor-in-chief, Alasdair Matheson, at amatheson@advanstar.com
References
(1) N. Gilart, F. Borrull, N. Fontands, and R.M. Marce, Trends Environ. Anal. Chem. 1, e8–e18 (2014).
(2) A. Spietelun, T. Marcinkowski, M. de la Guardia, and J.J. Namiesnik, J.Chromatogr. A1321, 1–13 (2013).
(3) B.K. Matuszewski, M.L. Constanzer, and C.M. Chavez-Eng, Anal. Chem.75, 3019–3030 (2003).
(4) K.A. Schug, "The LCGC Blog", 7 Jan 2013, www.chromatographyonline.com

Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.














