Developing Better GC Methods — A Blueprint
Pages 678
An excerpt from LCGC's e-learning tutorial on developing better GC methods at CHROMacademy.com
To obtain sensitive, robust, and reproducible gas chromatography (GC) methods, each stage of the chromatographic process needs to be carefully considered and optimized. It is also important to record and report as much detail within the method specification so that the method can be reproduced between operators, instruments, and laboratories. Table 1 represents a "blueprint" method specification with all of the information that is necessary to faithfully specify and reproduce a split–splitless GC method. Table 2 provides a blueprint starting point for the method development of a sample with unknown composition, but known to contain "trace" target analytes. Even if you are not developing methods - check the blueprint specifications against your GC methods. Do your methods contain all of the necessary details?

Table 1: Requirements for a properly specified splitless gas chromatography method with flame ionization detection (FID).
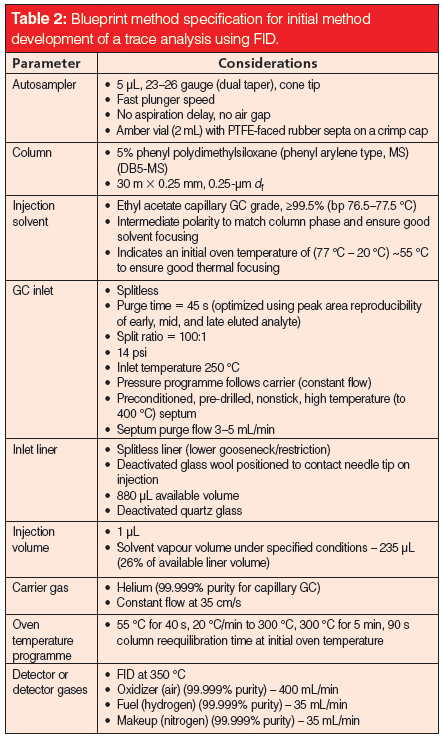
Table 2: Blueprint method specification for initial method development of a trace analysis using FID.

Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Determining the Serum Proteomic Profile in Migraine Patients with LC–MS
April 17th 2025Researchers used liquid chromatography–mass spectrometry (LC–MS) in their proteomic analysis to compare the serum proteome of migraine patients with healthy controls and to identify differentially expressed proteins as potential migraine biomarkers.














