Surfactant-Mediated Extractions, Part II: Coacervative Extraction and Related Techniques
LCGC North America
CAE is a versatile sample preparation tool for aqueous samples.
In a previous installment, we discussed cloud-point extraction (CPE) as an emerging technique for the isolation of organic compounds. In CPE, neutral surfactants are used at concentrations above the critical micelle concentration and phase separation is induced by increasing temperature. In coacervative extraction (CAE), ionic surfactants are used and phase separation is brought about by the addition of electrolytes or organic cosolvents, changes in pH, or other changes in solvent parameters. The CAE approach provides several advantages for organic extractions and may be used in a variety of formats. This month, we review the principles of CAE, its applications, and other sample preparation techniques based on the formation of coacervates.
Recall that surface active agents, or surfactants, are amphiphilic, organic molecules, possessing an ionic or polar head group attached with a long-chain hydrophobic tail. Considering the “like dissolves like” principle, the head groups interact with each other and with water or other polar solvents, while the hydrophilic tails are drawn toward each other or the nonpolar region of the system. For example, free fatty acids typically have surfactant characteristics; this is one reason why fatty samples often form emulsions during liquid–liquid extraction (LLE). At levels above what is known as the critical micelle concentration (CMC), these surfactant molecules form aggregates known as micelles.
The micellar structure can vary depending on the surfactant composition and concentration as well as solvent conditions, and may range from spherical to elongated to interconnected planar bilayers. The micelle may be described by the aggregation number, which is the number of surfactant molecules present in the micelle. Common examples include
- Micelles: Ball-like structures in aqueous solutions where the head groups form the surface of the ball, while the hydrophobic tails are hidden in the middle of the sphere.
- Reverse micelles: Similar to micelles, except found with nonpolar solvent systems. The head groups are inside the sphere and the hydrophobic tails are toward the outer surface.
- Bilayers: As in cell walls, generally the hydrophobic tails attract to each other and the head groups are on the outer surface of a sheet-like structure, resembling a film. Microemulsions: Single-phase dispersions of oil and surfactant distributed throughout an aqueous system (oil-in-water microemulsion) or water and surfactant distributed in an organic solvent (water-in-oil microemulsion). Rod-like micelles: Like micelles except the shape is extended into an elongated structure.
Coacervative Extraction
One commonly overlooked feature with these micellar structures is that they are in dynamic equilibrium with individual surfactant, solvent, and solute molecules continually entering and leaving the structure (1). Surfactant monomers move in to and out of the micelle aggregate on the order of microseconds (2). This equilibrium concept is important to understand when studying chemical separations involving micelles. In an aqueous system, hydrophobic interactions, such as London dispersion and other van der Waals forces, are primarily involved in solute partitioning into a micellar structure. Dipole–dipole and hydrogen bonding may also influence partitioning, and the possibility of multiple interactions is highly favorable for extraction purposes. This understanding leads to the development of coacervative extraction (CAE). In CAE, ionic surfactants are used and phase separation is brought about by the addition of electrolytes or organic cosolvents, changes in pH, or other changes in solvent parameters. Because of the electrostatic repulsion of oppositely charged micelles, phase separation is prevented. Changes in solvent properties that are less favorable for solubility cause self-assembly of these surfactants into micellar structures. Generally, these conditions can be considered dehydrating since aqueous systems are typically studied. This is called simple coacervation and is in contrast to cloud-point extraction (CPE), which uses neutral surfactants and a temperature-induced phase separation. This distinction is summarized in Table I. The addition of salt or cosolvents to induce coacervation is used with cationic surfactants, while coacervation of anionic surfactants is induced by changes in pH.

Upon separation of the two immiscible aqueous phases, the colloid- or micelle-rich phase is known as the coacervate layer, while the colloid-depleted, predominantly aqueous layer is called the equilibrium liquid. Note that each of these are distinct aqueous phases, the equilibrium layer contains greater than 99% water and the coacervate layer has up to 95% water. It is incorrect to think of coacervation as precipitation. Yazdi (1) reported that the colloidal region of the coacervate layer may form a sponge-like morphology. Anionic surfactants are most often used in CAE. Coacervates based on alkyl carboxylic acids demonstrate significant potential for CAE since protonation and deprotonation of the carboxylate can lead to a variety of intermolecular interactions favorable for extraction. Sodium dodecanesulfonic acid, sodium dodecanesulfonate, and sodium dodecyl sulfate are also widely used surfactants in CAE. During solubilization, solutes may partition into the micelle, intimately associating with the hydrophobic chains to the extent that solute molecules can be considered adjacent to these chains. If analyte solubilization occurs simultaneously with micelle formation, these solutes may form a separate phase between the surfactant layers. To visualize these situations, see reference 3 or the figures reproduced in a previous “Sample Preparation Perspectives" column (4). Ionic and polar solutes will associate near the ionic head groups in the surfactants used (that is, at the outer surface of the micelle); polarizable species will also associate somewhat close to the head group; small, nonpolar molecules can penetrate into the inner core of the micelle (2). The coacervation phenomenon dates back to more than 100 years ago, and a brief history is presented in Table II.
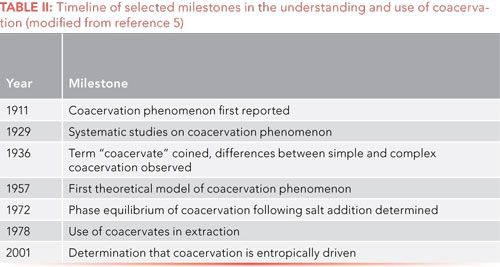
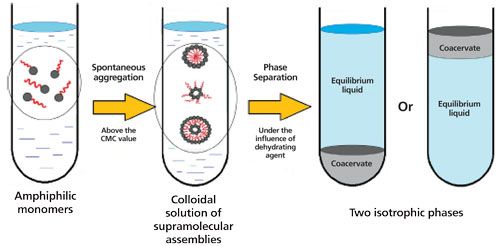
Although CPE was developed before CAE, the use of coacervates for extraction is growing dramatically and becoming as widely used as its counterpart. The advantages of CAE are plentiful. The technique is rather straightforward. Coacervates are nonflammable and nontoxic. Energy consumption is negligible and the use of organic solvents and other supplies is much less than in more traditional means of extraction, like LLE or solid-phase extraction (SPE), since the coacervate phase volume is often <500 µL with samples in the tens of milliliters. The extractions are rapid (on the order of 15 min) and the possibility of emulsion formation is negated. High extraction efficiencies with recoveries more than 90% can be obtained for a wide range of analyte polarities. The resulting coacervative layer is compatible with reversed-phase liquid chromatography (LC) and other analytical techniques, which means that the extracted material can be removed with a syringe and injected directly into the analysis instrument without sample cleanup or preconcentration. Perhaps the major drawback with CAE is that developed methods must often be adjusted with complex sample matrices, like plasma or urine, which contain high levels of salts. Thus, method development with CAE requires careful pH adjustment, followed by identification of the suitable temperature. Method development is completed with optimization of the surfactant concentration to balance sensitivity (low volumes desired) and accuracy and precision (high volumes desired) (1). Analyte enrichment may not approach the high values observed in some highly selective extraction techniques. Because coacervates are nonvolatile, gas chromatography (GC) is generally excluded for sample analysis. And, the complexity of the surfactant system may present detection issues. An overview of the CAE process is shown in Figure 1 and a much more rigorous description of the advantages and disadvantages of CAE can be found in the review by Melnyk, Wolska, and Namiesnik, especially their Table 4 (5).
Figure 1: Schematic diagram of coacervative extraction. (Adapted with permission from reference 5.)
Aqueous-Surfactant Two-Phase Extraction
A derivation of CAE is aqueous-surfactant two-phase (ASTP) extraction. In this mode, an equimolar mixture of cationic and anionic surfactants is used. This results in complex coacervation, dependent on the electrostatic interaction between the two oppositely charge surfactant molecules. The phase separation occurs in a limited range of concentrations that exceeds the CMC of both surfactants. The resulting micelle resembles a vesicle-like structure with a fluid-filled cavity surrounded by surfactant bilayers. The surfactant bilayers prevent intermixing of the water in the cavity with the bulk aqueous phase. When the appropriate concentrations are reached, micelle self-assembly occurs without requiring any additives. The extraction efficiencies can be quite high, depending on the surfactant compositions and concentrations (6).
Single-Drop Microextraction with Coacervates
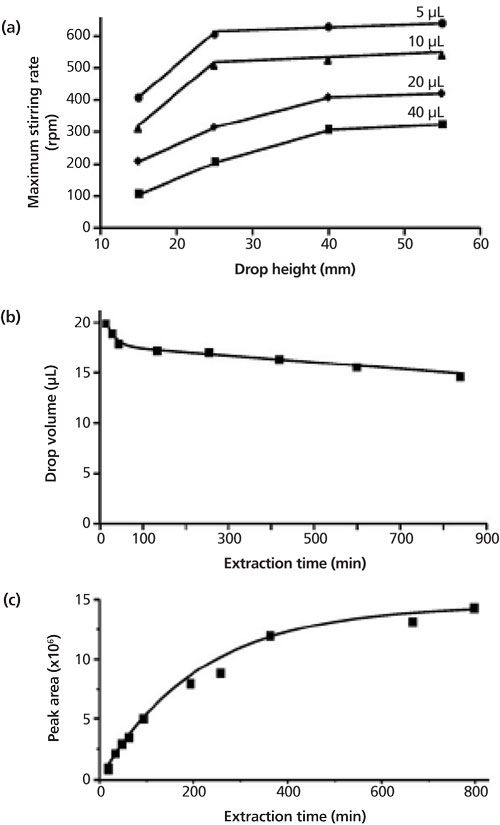
Single-drop microextraction (SDME) is seeing increasing interest because of the green benefits of the miniscule amount of extraction solvent used. One disadvantage of SDME is the instability of the hanging drop because of solvent volatility (in headspace applications), mutual solubility (in submersive applications), and low viscosity. Another mode of CAE uses a coacervate phase as the single drop, further reducing organic solvent use, utilizing a more volatility- and viscosity-friendly solvent, and, more importantly, providing a microextraction system with partition coefficients that is more favorable in some equilibrium-driven extractions. As alluded to previously, the coacervate phase is compatible with direct injection in reversed-phase LC. Water-immiscible coacervative phases in SDME are reported to be dependent on the density-dependent floatation, gravitation, and surface tension-related adhesive forces (4). Just as in organic-solvent SDME, the parameters of extraction time, drop volume, stirring rate, pH, and temperature influence both the equilibrium and kinetics of the extraction (7). The influence of these parameters is demonstrated in Figure 2. Perhaps one application where coacervates did not perform similarly to organic solvents in SDME is when the system is cooled. In the study cited (7), at temperatures below 15 °C, the coacervate drop began to gel, minimizing the extractive capability. Other variants of the SDME approach, like the floating drop systems, can also be used with coacervates.
Figure 2: Influence of the effects of (a) stirring rate, (b) drop volume, and (c) peak area on the performance of coacervative SDME. These effects are similar to that observed in SDME with organic solvent. (Adapted with permission from reference 7.)
Other Extraction Modes
Coacervates and related supramolecular systems are also used in a so-called supramolecular-based dispersive liquid–liquid microextraction (SM-DLLME) (2). The supramolecular system consisted of a dispersion of decanoic acid in an aqueous solution of tetrahydrofuran. After adding this solution to the sample, phase separation occurred by centrifugation. The tetrahydrofuran apparently was the dispersant, as in conventional DLLME, and induced the self-assembly of decanoic acid micelles.
Another extraction method involving surfactants, though not necessarily micelles, is as the stationary phase in SPE. Rubio and Perez-Bendito reported (8) the interaction of ordered structures of surfactants on metal oxides such as alumina, silica, titanium dioxide, and ferric oxyhydroxide to be used as an SPE sorbent phase. One particular advantage of this approach to SPE is the apparently high flow rates allowed during the sample loading step. The same research group (7) also used amphiphile-based supramolecular solvents for extraction of contaminants in liquid food products. The water-immiscible portion included nano-scale supramolecular aggregates, also of decanoic acid in aqueous tetrahydrofuran.
Discussion and Conclusions
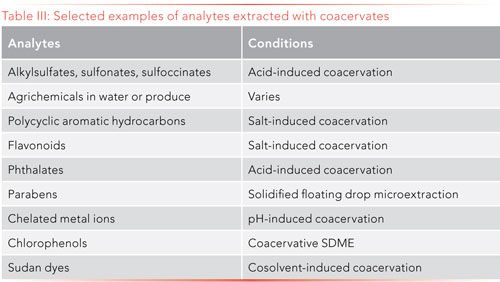
The wide selection of ionic surfactants available for CAE, along with the range of surfactant and solvent conditions that facilitate coacervation, make this approach a very versatile sample preparation tool for the extraction of aqueous samples. Table III presents a very brief overview to exemplify the types of analytes extracted. These examples tend to come from the environmental and food areas. Bioanalysis, and foods to a lesser extent, present more challenging method development because of the level of salts and the pH of the samples.
The use of surfactants in the extraction of aqueous samples, both CPE and CAE, presents a promising analytical approach. Over the next few years, we can expect an increasing report of these methods in the literature. The techniques are highly versatile, which should make them of interest to practically any analytical chemistry laboratory. What is holding these techniques back in their development is a lack of awareness. Since there is no instrumentation required and the use of supplies and reagents is minimal, we cannot expect vendors to promote CAE and CPE. Analytical textbooks do not update sample preparation topics frequently, if they are addressed at all, so rising analysts coming from the university system are not exposed to CAE and CPE in their general education. Now that significant applications and general reviews, such as those cited, are more frequent, the awareness is becoming available to make surfactant-based chemical extractions much more widely used. All laboratories currently doing LLE of aqueous samples are encouraged to check into these techniques to minimize the use of solvents and supplies (and the associated cost) and create faster and more efficient extractions.
References
- A. S. Yazdi, TrAc Trends Anal. Chem.30, 918–929 (2011).
- F. Merino, S. Rubio, and D. Perez-Bendito, J. Sep. Sci. 28, 1613–1627 (2005).
- A. Melnyk, J. Namiesnik, and L. Wolska, TrAC Trends Anal. Chem.71, 282–292 (2015).
- D.E. Raynie, LCGC North Am.34(1), 14–18 (2016).
- A. Melnyk, L. Wolska, and J. Namiesnik, J. Chromatogr. A1339, 1–12 (2014).
- P. Weschayanwiwat, O. Kunanupap, and J.F. Scamehorn, Chemosphere72, 1043–1048 (2008).
- F.J. Lopez-Jimenez, S. Rubio, and D. Perez-Bendito, J. Chromatogr. A1195, 25–33 (2008).
- S. Rubio and D. Perez-Bendito, TrAC Trends Anal. Chem. 22, 470–485 (2003).
Douglas E. Raynie

“Sample Prep Perspectives” editor Douglas E. Raynie is a Department Head and Associate Professor at South Dakota State University. His research interests include green chemistry, alternative solvents, sample preparation, high resolution chromatography, and bioprocessing in supercritical fluids. He earned his PhD in 1990 at Brigham Young University under the direction of Milton L. Lee. Raynie is a member of LCGC’s editorial advisory board. Direct correspondence about this column to LCGCedit@ubm.com.








Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.
Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.
