Supercritical Fluid Chromatography in the Pharmaceutical Industry: Implementation in Development and Quality Control
Special Issues
Supercritical fluid chromatography (SFC) is a well-established analytical technique used in the pharmaceutical industry for decades. However, it is still considered a new technique in some areas, for example, implementing the technique for purity profiling in late-stage development and production. In pharmaceutical analytical departments, SFC serves a wide variety of purposes, including compound purification, purity profile, and chiral analysis. Depending on the phase of drug development, the analytical performance requirements, such as speed of analysis, efficiency, and sensitivity, may vary considerably. The end goal is to provide robust, reliable and transferable analytical SFC methods. The challenges for future development and widespread implementation of SFC and the implementation of SFC in quality control (QC) laboratories using modern instrumentation are also discussed.
Supercritical fluid chromatography (SFC) is a well-established analytical technique used in the pharmaceutical industry for decades. However, it is still considered a new technique in some areas, for example, implementing the technique for purity profiling in late-stage development and production. In pharmaceutical analytical departments, SFC serves a wide variety of purposes, including compound purification, purity profile, and chiral analysis. Depending on the phase of drug development, the analytical performance requirements, such as speed of analysis, efficiency, and sensitivity, may vary considerably. The end goal is to provide robust, reliable and transferable analytical SFC methods. The challenges for future development and widespread implementation of SFC and the implementation of SFC in quality control (QC) laboratories using modern instrumentation are also discussed.
Product development life cycle in the pharmaceutical industry can be divided into three distinct phases: discovery, development, and registration or manufacturing. At each stage, supercritical fluid chromatography (SFC) has been implemented for several important applications.
Each of these phases has different requirements for performance attributes of separation techniques used to support product development. In the discovery phase, where very large numbers of compounds are screened against multiple targets, the emphasis is on selectivity and speed of analysis. For the next phase, where the stability of the active ingredient and formulation of the product are established, the emphasis shifts more towards high resolution and high sensitivity. This stage involves development of synthetic route and formulation development. The emphasis on high resolution and sensitivity is a result of the need to be able to separate a large number of components and quantification at low levels, for example, 0.05% by area. Finally, during preparation for registration application and transfer to manufacturing facilities, the key attribute of an analytical method is its focus on robustness, reliability, ease of operation, and transferability of the method. In the case of less popular analytical technology, such as SFC, the availability of instrumentation and relevant skill sets in quality control (QC) laboratories become additional requirements.
The intrinsic nature of supercritical CO2, with its low viscosity, high density, and relatively low toxicity (compared to other supercritical substances), makes it an ideal mobile phase for certain types of chromatographic applications. Amongst these, it is known to be very efficient for fast separations as well as isolation and purification of compounds. Both of these applications are well established in the drug discovery phase (1,2). For example, the use of carbon dioxideâbased mobile phases for preparative chromatography allows clean and rapid recovery of the purified compounds, with dramatic savings of organic solvent, energy, time, and overall cost (3). Another attractive feature of SFC is a higher success rate in chiral method development when compared to other chromatographic modes (normalâphase or reversedâphase LC) (4,5). This makes it the technique of choice for many chiral separations (6,7).
Despite the introduction of commercial instruments in the 1980s, in the development and subsequent phases of drug development, SFC is still regarded primarily as a research tool rather than a routine technology. Consequently, SFC methods are rarely implemented throughout later stages of development and are, equally, rarely used in registration applications.
Important advances in understanding and controlling SFC separations for purity profiling have been made in the past 15 years: the high efficiency and the modelling of selectivity were fundamental in determining the potential and limitation for using SFC for purity profiling (8–14).
The relatively slow penetration of SFC into the manufacturing QC area was mainly a result of real, or sometimes perceived, negative user experiences with former instrument reliability, complex technology transfers, and lack of method robustness.
In this article, we assess the transferability of an SFC method across modern SFC instruments from three different vendors with the aim of assessing some of the performance attributes relevant to registration application activities and transfer to manufacturing environments, rather than comparing the performance of the instruments.
Experimental
Instruments and Methods: System 1 was equipped with an autosampler with a 10-µL loop and a diode array detector (DAD) with a 10-mm high pressure flow cell.
System 2 was equipped with an autosampler with a 100-µL loop and a DAD with a 3-mm high pressure flow cell.
System 3 was equipped with an autosampler with a 5-µL loop and a DAD with a 10-mm high pressure flow cell.
Chiral Method 1: The column used was a 150 mm × 3.0 mm, 3-µm Chiralcel IC-3 (Chiral Technologies Europe) (column 1). HPLC-grade methanol with 10 mM ammonium formate (Fisher) was used as organic modifier, 1.9 mL/min flow rate. Gradient programme: 5% (hold 0.5 min) to 20% in 30 min, then to 45% in 35 min. Outlet pressure was set at 105 bar, temperature was 50 °C. Sample was prepared at 1.0 mg/mL in methanol. Detection was set at 275 nm with compensation reference from 310 nm to 410 nm on instrument system 1.
Chiral Method 2: The column used was a 450 mm × 4.6 mm, 3-µm Chiralcel IC-3 (Chiral Technologies Europe) (column 2), organic modifier was HPLC-grade methanol, 3.0 mL/min. Gradient programme: 2% (hold 1.0 min) to 34% in 32 min. Outlet pressure was set at 120 bar, temperature was 25 °C. Sample was prepared at 2.0 mg/mL in dichloromethane. Detection was set at 232 nm, with reference from 440 nm to 540 nm (injection volume was 10 µL on system 1, 30 µL on system 2 to compensate for a shorter DAD flow cell, 5 µL on system 3 in full loop).
Discussion
For SFC to be considered a technique to support registration applications and be transferred to a manufacturing environment, it must satisfy certain attributes that fall into four broad categories:
- Applicability
- Robustness
- Validation
- Transferability.
Applicability: During method development, it must be demonstrated that SFC offers clear advantages over more established techniques, such as LC. This could be, for example, better resolution, better peak shape, and better compatibility with sample components (stability). The field of SFC applications is expanding and it covers a wide range of chemical structures with different polarities and applies to structurally similar compounds, such as stereo- and positional isomers (15–19). In drug development laboratories, SFC has become a technique of choice for chiral separations, when orthogonal selectivity is required, but also for the separation of compounds that are unstable or insoluble in aqueous solutions (20–22).
For example, a normal-phase LC method successfully implemented in a QC laboratory was used for the chiral analysis of an intermediate in the synthesis of a new product. This method, however, exhibited some challenges, such as very long equilibration time and the use of the mobile phase containing n-hexane (safety implications) and HPLC-grade ethanol (cost, a controlled solvent, and not always readily available). A newly developed chiral SFC method (chiral method 2) offered several advantages: first, a straight linear gradient with pure methanol as mobile phase modifier; second, instrument and chromatographic separation setup was much simplified, resulting in easy operation; and, finally, improved resolution of all potentially interfering compounds maximized method robustness. An added advantage was the compatibility with mass spectrometry (MS), which allowed simple peak identification.
Robustness: During robustness testing, the ability to separate and quantify required components must be shown and should remain unaffected by changes in operating parameters. This must also be true for chromatographic consumables, such as batch-to-batch column variability.
In order to achieve maximal method robustness, the development had to be approached in a systematic manner as detailed below:

Column and Mobile Phase Choice: The most suitable column should be chosen in a systematic approach, usually achieved through screening a small number of columns with different selectivities. This must be performed together with mobile phase selection because the latter also has a significant impact on resolution. In this case column 1 was selected as the chiral selector because it provided optimal peak symmetry and higher resolution with methanol as organic modifier. When it comes to mobile phase selection, simple composition is preferred where possible. Ammonium formate was used as an additive to methanol. Ammonium formate could be used instead of a isopropylamine and trifluoroacetic acid mixture that is often used during column screening.
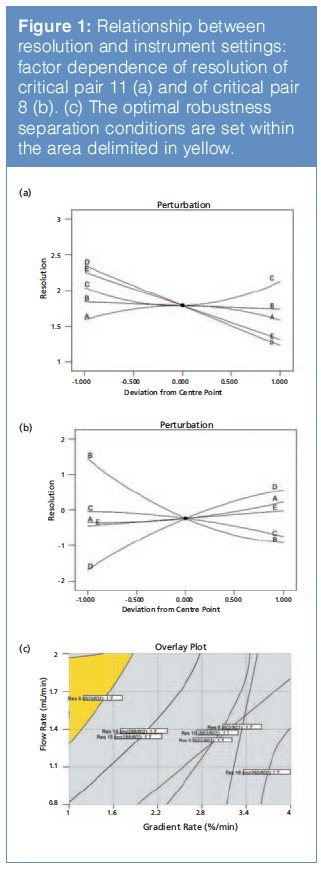
Separation: For separation development, a sample mixture that contains all relevant components that may interfere with the analytes of interest must be used if available. A multifactorial design of experiments (DOE) with three levels of response surface (26 runs plus 3 centre points) was chosen to model the retention and separation of each component (retention time and resolution). Modelling the relationship of retention and resolution from DOE experiments (Figures 1[a] and 1[b]) allows selection of optimal operating parameters to maximize method robustness (Figure 1[c]).
Detection: In order to achieve optimal sensitivity and reduce baseline noise, UV detection settings must be optimized together with consideration for sample loading and its impact on peak shape and resolution.
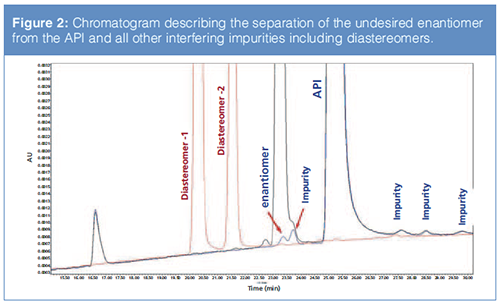
Figure 2 shows the relative chromatogram obtained (Chiral Method 1).
Validation: Any new technology used to support registration applications must meet established validation criteria, for example, ICH Q2 R1. There are numerous literature reports describing that SFC under optimal operating conditions is perfectly capable of achieving this requirement (23,24).
Transferability: Before using an analytical application in a manufacturing environment, it must be transferred to a receiving laboratory. This is typically performed under a standard analytical transfer protocol. In the case of SFC, suitable instrumentation and appropriate skills for operation must be available at the receiving laboratory.
A recent study tested the performance of a method for the impurity control of salbutamol in 19 different laboratories. The method was run on a single technology platform and the statistical analysis of the quantitative results reported reproducibility equivalent to LC methods (25).
To demonstrate the suitability of modern SFC instruments, the chiral method described previously (see “Applicability” section), which was developed on System 1, was used. Validation criteria, which are often affected by instrumentation, such as specificity, sensitivity, and precision, were used to demonstrate performance and suitability of all of these systems.
Specificity: It was demonstrated that the specificity of the chiral SFC method described in “Applicability” section (Chiral Method 2) was preserved when this method was replicated on three different columns and three different instrument platforms (Figure 3). We believe this was a direct consequence of systematic method development.
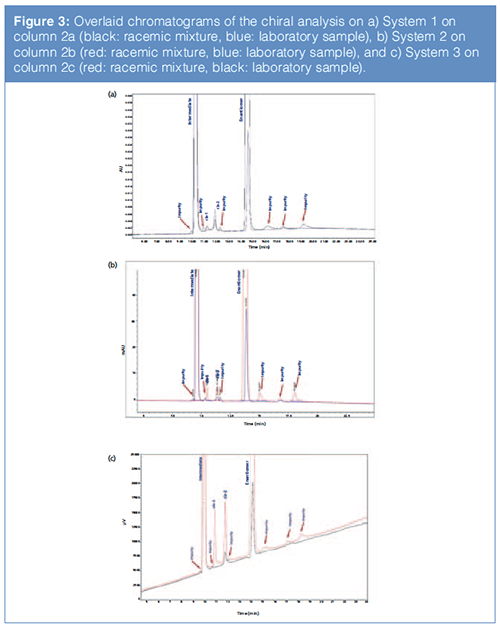
Figure 3 shows the overlaid chromatograms of the chiral analysis of the racemic mixture and a laboratory sample analyzed on (a) System 1 on column 2a, (b) on System 2 on column 2b, and (c) on System 3 on column 2c.
Sensitivity: The reporting limit (set at 0.05% of the nominal concentration) needs to be higher than the limit of quantification (LOQ), which is satisfied with a signal-to-noise (S/N) ratio >10 or with a RSD <10% on at least six injections.
In Table 1, we report the average S/N values and the RSD% on area on six repeated injections, for both intermediate (main) and enantiomer peaks. System 1 showed higher values of S/N values compared to other systems for this analysis (The method was first developed on System 1). Lower S/N values were obtained on System 2, however, RSD% on area were comparable and were satisfactory with suitability requirement (<10%). Also, on System 3 lower S/N values were obtained compared to System 1: it must be noted, though, that the sample loop on System 3 was 5 µL, which means that the column loading was half compared to System 1 and System 2 (10 µL as per method). Also on System 3 RSD% on area were lower than 10% and therefore satisfied the suitability requirement (<10%). The data demonstrate that all instruments are capable of performing analysis compatible with GMP requirements.

Precision: Precision is one of the system suitability tests necessary for GMP applications and is therefore one of the most important parameters to evaluate modern SFC instruments.
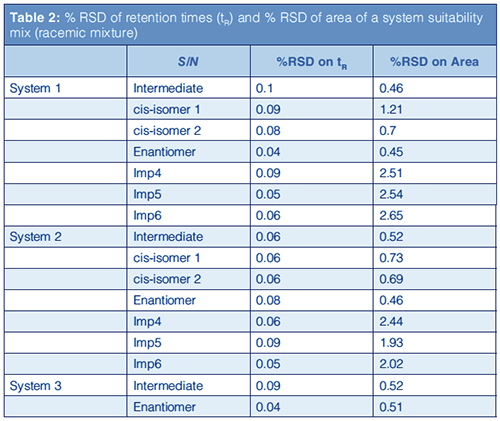
The reported values of RSD% for retention time and area on the analysis of the racemic mixture are provided in Table 2. Figure 4 shows the overlay of ten repeated injections of a laboratory sample on three different vendors’ instruments: (a) System 1, (b) System 2, and (c) System 3.
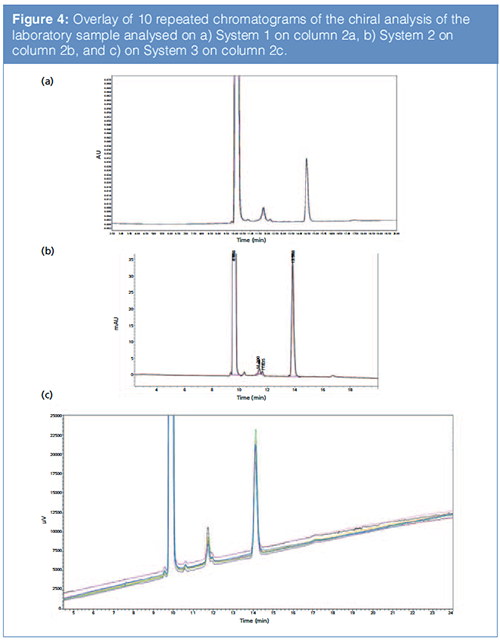
The data show comparable values on all instruments, with RSD% of area around 0.5% for both intermediate and undesired enantiomers (NB: for comparison only, Table 2 provides additional data relative to processârelated impurities [PRIs] present in the sample. These were not quantified with the described method, but on a dedicated reversed-phase LC method).
Because of difference in performance, primarily sensitivity, it is not guaranteed that a method that is developed on one system can be successfully transferred to a system from a different vendor, or with a different configuration or setup. It is recommended that development and transfer are planned and performed on the same technology platform. Otherwise, it is highly recommended to introduce the necessary adaptation to the method (for example, a higher sample concentration) to ensure successful transfer on a different instrument at the receiving laboratory. The knowledge of the instrument vendor or configuration at the receiving end is essential. The necessary modification to the method should be already accounted for during the development phase. This approach is no different than for any other LC method.
Implementation New Analytical Technology in a Manufacturing Environment: Equipping a QC laboratory with a “new” technology has financial implications: not only the initial capital expenditure, but also long-term service, maintenance, and running costs need to be budgeted. These costs need to be offset against the number of samples that will be run by SFC throughout the year. Any instrument fault may generate a downtime that could represent a bottleneck when release of product is due and may result in a delay. This creates much more of an impact when there is only one instrument available. The availability of multiple systems may not be justified or financially viable for a relatively low utilization of SFC, compared to other technologies.
However, the return on investment resides with a faster sample turnaround as a result of increased simplicity and speed of the method, and lower instrument downtime as a result of a more robust application.
The implementation of SFC into a laboratory needs to take into consideration the laboratory infrastructure. The delivery of CO2 needs to be risk assessed and this should include evaluation of the supplier, the storage and handling of the cylinders and pump (for external supplies), and the location of the instrument to ensure adequate venting (fume hood or air circulation).
Staff training is probably one of the most important aspects that needs to be taken into account when equipping a laboratory with SFC. Adequate training is provided by most vendors and analysts are quickly enabled to use the instruments and run a method as received. Most modern systems have automated start-ups and are controlled by software that analysts may already be familiar with if they are used to running LC.
Troubleshooting may be more complicated at first because of unfamiliarity with the technique, but this will become simpler with time and greater experience with the technique. Support from vendors and subject matter experts is essential to facilitate the initial implementation and longer term running of the technique. Effective training and support should mean that SFC will become no different to LC in its longer term routine application.
Conclusions
Modern SFC technology is suitable for QC analysis in regulated environments. Systematic science-based method development is essential to ensure that SFC methods successfully pass validation and transfer to QC laboratories. In particular, SFC should be selected for the right application where it provides a higher degree of speed, robustness, and simplicity over alternative or more established techniques.
Method transfer among different instrument platforms carries risks (as with any technology). It is advisable to know the differences in the instruments (for example, sensitivity, instrument dispersion, as well as operating parameters and limits typical of each system, such as temperature range, flow, and pressure limits) and to anticipate these differences during development to forecast the necessary adaptations, such as injection volume, and sample concentration.
The value and the challenge in the implementation of SFC in a QC laboratory go beyond the performance of a method or an instrument and associated financial commitment, the investment has to include adequate and appropriate staff training.
The development of an SFC method and its transfer to QC laboratories is justified when it responds to the demands of robustness, for example, in the case of chiral analysis and the separation of water-insoluble or unstable solutes.
Initially, achiral SFC analyses may be less common in QC laboratories, with LC remaining the preferred technique. SFC has a higher chance of offering a robust method when it is a simpler approach to solving an analytical problem, whether through simpler mobile phase preparation, or rapid separation without the need for complex mobile phase gradients and lengthy system setup.
For good reasons, more and more contract research organizations (CROs) are equipping their analytical laboratories with SFC instruments and investing in staff training to enable it to be run on a routine basis to satisfy the increasing demand from pharmaceutical research and development. A closer interaction with CROs equipped for SFC analysis represents an opportunity for pharmaceutical industry, provided the necessary qualification and data integrity requirements are maintained.
Acknowledgements
We thank David Mason and Concept Life Sciences for the collaboration with executing part of the Transferability experiments.
References
- T.W. Farrell, C. Aurigemma, and D. Masters-Moore, J. of Liq Chromatography & Related Technologies32(11–12), 1689–1710 (2009).
- T. Zelesky, American Pharmaceutical Review 11(7), 56–62 (2008).
- E. Francotte, LCGC Europe29(4), 194–204 (2016).
- K. DeKlerck, D. Mangelings, and Y. Vander Heyden, J. Pharm. Biomed. Anal.69, 77–92 (2012).
- S. Khater, M. Lozac’h, I. Adam, E. Francotte, and C. West, J. Chromatogr. A1467, 463–472 (2016).
- M. Maftouh, C. Granier-Loyaux, E. Chavana, J. Marini, A. Pradines, Y. Vander Heyden, and C. Picard, Journal of Chromatogr. A1088(1–2), 67–81 (2005).
- G. Terfloth, Journal of Chromatogr. A906(1–2), 301–307 (2001).
- C. Brunelli, Y. Zhao, M. Hannah-Brown, and P. Sandra, J. Chromatogr. A1185, 263–272 (2008).
- C. Brunelli, Y. Zhao, M. Hannah-Brown, and P. Sandra, J. Sep. Sci. 31, 1299–1306 (2008).
- C. West, J. Ogden, and E. Lesellier, J. Chromatogr. A1216, 5600–5607 (2009).
- E. Tyteca, V. Desfontaine, G. Desmet, and D. Guillarme, J. Chromatogr. A1381, 219–228 (2015).
- C. West, J. Melin, H. Hansouri, and M. Mengue Metogo, J. Chromatogr. A1492, 136–143 (2017).
- L. Novakova, A.G.G. Perrenaud, I. Francois, C. West, E. Lesellier, and D. Guillaurme, Analytica Chimica Acta824, 18–35 (2014).
- C. West, E. Lemasson, S. Bertin, P. Henning, and E. Lesellier, J. Chromatogr A1440, 212–228 (2016).
- V. Desfontaine, D. Guillarme, E. Francotte, and L. Nováková, J. Pharm. Biomed. Anal.113, 56–71 (2015).
- T.A. Berger, J. Chromatogr. A 785(1–2), 3–33 (1997).
- M.A. Patel, F. Riley, J. Wang, M. Lovdahl, and L.T. Taylor, J. Chromatogr. A1218(18), 2593–2597 (2011).
- L. Taylor, Anal. Chem.80(12), 4285–4294 (2008).
- I. Brondz and A. Brondz, Int. Journal of Analytical Mass Spectrometry and Chromatography2, 15–24 (2018).
- N. Gibitz Eisath, S. Sturm, and H. Stuppner, Planta Med84, 361–371 (2018).
- K. TyÅkievitz, A. Debczak, R. Gievsztor, T. Szymczak, and E. Rój, J. Sep. Sci. 41, 336–350 (2018).
- L.T. Taylor, Journal of Supercritical Fluids47(3), 566–573 (2009).
- C. Brunelli, M. Dunkle, S. Morris, and P. Sandra, Chromatography Today (15 Oct 2009).
- B. Andri, P. Lebrun, A. Dispas, R. Klinkenberg, B. Streel, E. Ziemons, R.D. Mariani, and Ph. Hubert, J. Chromatogr. A1491, 171–191 (2017).
- A. Dispas et al., Journal of Pharmaceutical and Biomedical Analysis 161, 414–424 (2018).
Claudio Brunelli is principal scientist at Pfizer R&D LTD, Analytical R&D in Sandwich, UK. He completed his Ph.D. in analytical chemistry at the Universita’ degli Studi di Torino in Italy, with Prof. Carlo Bicchi. In 2004, he became a Pfizer Postdoc Fellow at Pfizer Analytical Research Centre (PARC) in Belgium, under the supervision of Prof. Pat Sandra on high resolution supercritical fluid chromatography (SFC) for pharmaceutical analysis. He joined Pfizer Analytical Research and Development in Sandwich UK, in 2007.


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)