Spring Cleaning in the Laboratory
LCGC Asia Pacific
The regular maintenance of gas chromatography components, including symptons, causes and remedies.
Laboratory instrument systems require regular maintenance for consistent and reliable results. In this month's "GC Connections", John Hinshaw addresses the regular maintenance of gas chromatography components that can become compromised in the course of normal use, including symptoms, causes and remedies.
Periodic maintenance is the mainstay of keeping laboratory instruments running at the peak of performance. Analysts can avoid many problems by proactive evaluation, repair and replacement of critical system components on a regular basis. The alternative of allowing contamination to build up, syringes to wear out, or septa to leak only results in increased down-time compared with a planned maintenance programme. Unplanned maintenance and repair is not predictable and, in my experience, most incidents such as this occur at the worst time.
Periodic maintenance procedures fall into two categories. First are those that should occur on a regular basis, for example, every six months. Second are those for which the frequency is determined by system usage characteristics (for example, to be performed every 200 injections) and usually set by the type of sample and its level of contamination; dirtier samples impose more frequent maintenance on syringes, inlets and the column entrance or retention gap. This instalment of "GC Connections" presents some recommended maintenance intervals and related suggestions for a number of the major components of a gas chromatography (GC) system. Table 1 lists maintenance items and both regular intervals as well as sample-based intervals. The suggestions in this column are not intended as a set of rules to be followed rigidly. Rather, the information should be considered as a partial list of items that should be subject to periodic maintenance. Each laboratory has different requirements, not all of which appear here. It's a good idea to review maintenance procedures as laboratory requirements and usage patterns change over time.
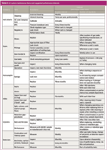
Table I: GC system maintenance items and suggested performance intervals.
Regular Timed Maintenance
Procedures that should be performed on a regular time basis include such simple things as straightening up the laboratory area, dusting behind instruments, archiving instrument logbooks, or defragmenting disk drives. Most laboratories run a fairly clean operation, although I've seen some remarkable exceptions in my travels. Usually, some dirt and grime will accumulate beneath instruments and computers or become trapped around gas lines and electrical cords. More seriously, a build-up of dirt on instrument cooling air intake vents and filters, on internal cooling fans and heat sinks, and around computer components eventually can compromise instrument operation. While it's simple enough to clean around the outside of instruments and computers, opening up the panels and clearing out the internal dust is another matter. Doing so exposes the inside of the instrument, or computer, to potential harm from static discharges, often found at the end of a vacuum cleaner hose, and also exposes untrained personnel to high voltages that might be present, in some cases even if the power is off and the AC line unplugged. Such operations are best left to trained service persons.
Certain pieces of equipment should be checked — and checked functionally where appropriate — every so often. Gas tank regulators, external gas lines and their fittings, gas generation equipment and the types or grades of gas in use should all be examined at least every three months. Verify that the carrier and detector gases are of the correct identity and grade. I once came upon a laboratory where the helium carrier gas cylinder had mistakenly been replaced with a nitrogen cylinder (in the US both have the same style high-pressure fitting), which resulted in some apparently mysterious chromatographic behaviour until the problem was discovered.
Leaks can develop in gas fittings as they are opened and resealed — for example, while replacing in-line gas filters. And speaking of gas filters, it's a good idea to label new filters as they are installed with the date and type of filter being put into use. Check each in-line gas union with a high sensitivity helium leak detector — they also react to hydrogen. Such a device won't pick up leaks in nitrogen or air lines, but I don't recommend using any kind of liquid leak solution. In these cases, a pressure drop test will reveal any gross leaks. Turn off the air or nitrogen carrier, detector, or make-up gas flow at each connected gas chromatograph, then close the gas tank high-pressure side regulator valve and wait 10–20 min. When the high-pressure valve at the tank is reopened, observe whether the high-pressure gauge reading jumps upward significantly. If so, then there is a downstream leak somewhere. I sometimes will repressurize the lines with helium just so that I can use the helium leak detector to pinpoint such a leak. Just remember to reconnect to the correct tank when you are ready to put the instrument back into service.
Gas generation systems are easy to install but sometimes, especially when installed out of direct view, they can be ignored for longer than desirable. I have seen several instances of gas generation equipment running with the "service required" and operational indicators facing the wall, presumably to get better access to the inlet and outlet fittings, so that it was impossible to see the status of the generator. Eventually, such situations will manifest themselves with increased noise and drift. It's preferable to check and perform the necessary maintenance in advance.
The high-pressure diaphragm in the dual-stage pressure regulators used in chromatography laboratories operates under a high stress level at the intermediate pressure stage of up to 500 psi (345 N/cm2). With time and many pressure/flow changes during normal use the high-pressure diaphragm can suffer metal fatigue, as can the counter-spring. This gradual loss of performance can take years, but then again, I've seen many pressure regulators that must be more than 25 years old still in routine use in GC laboratories. Early symptoms of regulator degradation can be a loss of regulating ability, so I like to test regulators every year or so by turning off the gas at the regulator outlet valve and then disconnecting the supply tubing somewhere convenient toward the instrument. Then I gradually turn on the gas at the regulator outlet valve to increase the flow as I observe the pressure on the outlet gauge. As the flow rate increases, the outlet pressure should drop slightly but remain within 5 psi or so of a 90 psig (620 kPa) set-point for a high-quality, dual-stage laboratory regulator. Another sign of a problem is when the outlet gauge does not return to zero when the regulator is depressurized, or if the needle is bent. Such a broken regulator should be replaced immediately.
Pure inert gas supply tanks generally don't have an expiration date, but certified gas standards do. I sometimes will keep expired gas standards around for a quick test of a set-up, but I always make sure that in-certification tanks are employed when making quantitative measurements. The same goes for liquid standards. Also, any gas blending equipment in use will have a fixed expiration date for its accuracy certification and will need to be recalibrated regularly, usually once a year. Other equipment in use in the laboratory such as precision thermometers, volt-ammeters and data-acquisition systems also require recalibration on a regular basis. GC oven temperatures sometimes drift out of specification over a long time period — more so on older instruments — and many laboratory periodic maintenance procedures call for checking and recalibration. Also, electronic pressure control (EPC) systems will require regular zeroing and accuracy checks as the electronic pressure gauges contained in them undergo normal ageing and drifting.
Sample-determined Maintenance Intervals
Beyond fixed maintenance intervals, some maintenance procedures should occur on the basis of how many injections have transpired and also in relation to the level of sample cleanliness. For example, septa develop leaks in proportion to the number of injections, while autosampler syringe barrels wear both with the number of fill–eject cycles and the level of sample contamination. Many of these items should be performed on a "whichever comes first" basis and these have entries in both columns in Table 1 labelled "Regular Interval" and "Number of Injections."
Autosamplers include components in both categories. GC system operators should verify the basic mechanical functions of an autosampler on a regular basis by observing that the device runs smoothly, triggers no errors and delivers expected levels of precision and accuracy. A sudden loss of performance in a GC system might be a symptom of a poorly functioning autosampler. Autosampler syringes, however, are subject to wear, both as a function of how many fill–eject cycles occur plus how much residue is in the samples. Counter to intuition, a larger number of postinjection wash cycles can wear out a syringe in fewer total injections, if the sample contains non-volatile residue that builds up inside the syringe barrel. Selection of the right rinse solvent can help increase syringe life significantly. Leak testing a syringe involves dismounting it from the autosampler, filling and emptying the barrel with a representative solvent while observing any bubble formation or leaks around the needle or barrel, and assessing the difficulty with which the plunger moves. If any doubt exists as to the condition of a syringe, it should be replaced.
Inlet septa don't degrade over time, but they can develop significant leaks fairly rapidly, often after as few as 100 injections. This depends strongly upon the type of syringe needle and whether automated or manual injections are performed. Inlet liners, however, are subject to degradation mostly from the build-up of sample residue that can interact with subsequent samples either chemically or by adsorption. Replacement intervals for liners run from one injection to thousands. I have come across a number of laboratories that perform little to no sample preparation of biological samples, thus finishing off one liner per injection, as well as others, in the business of checking highly pure solvents for impurities, where one liner can last for a very long time.
With elevated inlet temperatures, the o-ring seal at the top of the liner does degrade over time, so even in cases in which the liner itself is still fine, the seal should be replaced at least on a regular timed interval. In inlet systems with a lower metallic wafer seal, if the seal is interrupted for any reason, then it must be replaced because leak-tight metal-to-metal contact requires a pristine surface. It's also a good idea to double-check split vent and septum-purge flows after changing any injector parts or the column; those steps should be part of a normal method set-up procedure as well.
The column itself is subject to degradation resulting from sample injections as well as temperature programming. Dirty samples can deposit nonvolatile residues at the beginning of the column, which leads to increased column bleed, adsorption with associated peak tailing and the potential breakdown of sensitive compounds in subsequent injections. Other column-degradation routes include oxygen in the carrier gas from poor supplies or leaking fittings. In any case, the damage is cumulative across multiple injections and temperature programme cycles. Regular evaluation of column performance will track any degradation and provide a clear indication of when to change the column. A retention gap or precolumn in front of the analytical column will trap much of the sample residue so that trimming or replacing the retention gap section will restore much of the original column performance. However, a retention gap will do nothing to prevent thermal damage to a column.
Detectors will also degrade over time and as a result of the accumulation of column bleed by-products. Symptoms include increased noise, reduced sensitivity and higher offsets. Detector components such as flame ionization jets and collectors, photoionization and flame-photometric optical windows, and thermionic beads all require regular inspection, cleaning, or replacement as appropriate. And like inlet and column flows, analysts should check detector flows after performing any maintenance procedures to be sure that normal flow rates have been reestablished.
Spring Cleaning
Laboratories can benefit greatly from enacting regular maintenance procedures that are triggered by both timed intervals and by the sampling history of individual instruments. Initially, establishing a regime for sample-based maintenance means monitoring the relationship between the number of injections, sample purity and type, and the changes in instrument performance that result. The initial effort can be well worthwhile and can help reduce instrument downtime as well as increase results quality. "Spring cleaning" is not really a good term to describe this process. It implies a once-yearly effort to clear out long accumulated dust and debris, while the concept discussed here is a proactive effort to establish regular and directed procedures that will prevent the "build-up" of performance-robbing conditions in laboratory gas chromatographs.
"GC Connections" editor John V. Hinshaw is senior research scientist at Serveron Corp., Hillsboro, Oregon, USA and a member of LCGC Europe's Editorial Advisory Board. Direct correspondence about this column should go to "GC Connections", LCGC Europe, Poplar House, Park West, Sealand Road, Chester CH1 4RN, UK or e-mail the editor, Alasdair Matheson at amatheson@advanstar.com















