Automated USP and EP GPC/SEC Analysis of Low Molecular Weight Heparin (LMWH)
LCGC Asia Pacific
PSS Application Note
Daniela Held, PSS Polymer Standards Service GmbH, Mainz, Germany.
Introduction
LMWHs are defined as heparin salts having an average molecular weight of less than 8000 Da and for which at least 60% of all chains have a molecular weight less than 8000 Da. GPC/SEC is the method of choice to measure the molecular weight (molar mass) and the molecular weight fractions above/below a molecular weight limit. Both, the Pharmaeuropa (EP) and the US Pharmacopeia (USP), require GPC/SEC to characterize commercially available LMWHs.
Results
Although the calibration procedures described in the EP and USP are totally different, a dedicated GPC/SEC software can be used to analyse the heparin data in accordance with both standards precisely. Figure 1 shows the final results for the analysis of unknown low molecular weight heparin. PSS WinGPC Unity GPC/SEC software allows 2 markers to be set at 2000 and 8000 Da, to display the measured percent below 2000 Da, above 8000 Da and in between these values.
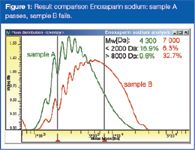
Figure 1
Of utmost importance is the y-axis of the result window. WinGPC Unity shows a true molar mass distribution with a w(log M) axis. Only this guarantees correct fractions above/below the molecular weight borders 2000 and 8000.3

Table 1: Comparison of experimental conditions from USP and EP for the characterization of LMWH.
References
1. USP Enoxaparin sodium http://www.usp.org/pdf/EN/USPNF/enoxaparinSodium.pdf
2. EP Low-Molecular-Mass (LMM) heparins Monograph 0828.
3. D. Held and P. Kilz; Quantification in LC and GC - A Practical Guide to Good Chromatographic Data [H.-J. Kuss/ S. Kromidas (eds.)], 271–302, Wiley-VCH, Weinheim (2009).
PSS Polymer Standards Service GmbH
In der Dalheimer Weise 5, D-55120 Mainz, Germany
tel. +49 6131 96239 41 fax +49 6131 96239 11
E-mail: DHeld@polymer.de Website: www.polymer.de

Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.
Investigating the Protective Effects of Frankincense Oil on Wound Healing with GC–MS
April 2nd 2025Frankincense essential oil is known for its anti-inflammatory, antioxidant, and therapeutic properties. A recent study investigated the protective effects of the oil in an excision wound model in rats, focusing on oxidative stress reduction, inflammatory cytokine modulation, and caspase-3 regulation; chemical composition of the oil was analyzed using gas chromatography–mass spectrometry (GC–MS).













