The Separation of Enantiomers on Modified Cyclodextrins by Capillary Electrochromatography (CEC)
Various modes of capillary electrochromatography (CEC) for the separation of enantiomers on immobilized cyclodextrin derivatives are described. The following techniques have been used: (i) open-tubular electrochromatography (o-CEC), (ii) packed electrochromatography (p-CEC) and (iii) monolithic electrochromatography (rod-CEC). Three different strategies to prepare enantioselective cyclodextrin-coated chiral monoliths are described. The advantages and disadvantages of the various methods are outlined.
The chromatographic separation of enantiomers constitutes an indispensable tool in contemporary research concerned with chirality ("handedness"). Research in modern enantiomeric analyses is widespread in many scientific fields (Figure 1). The versatility of nonracemic chiral stationary phases (CSPs) for enantioseparations has been demonstrated by gas chromatography (GC), followed by liquid chromatography (LC) and supercritical fluid chromatography (SFC) and, more recently, by capillary electrochromatography (CEC). Modified cyclodextrins (CDs), although only available in the all-D-form, play a pivotal role in enantioselective electromigration methods. CDs are employed either as chiral mobile phase additives (CMPAs) in capillary electrokinetic chromatography (CEKC)1 or as chiral stationary phases (CSPs) in CEC.2–4 The use of a chiral selector as CSP in CEC has the following merits: (i) very economic use of the chiral selector, (ii) straightforward hyphenation with mass spectrometry, (iii) no run-to-run capillary rinsing procedures and (iv) ease of analyte detection in the absence of the chiral selector. In principle, chiroptical detectors and multidimensional approaches can also be envisioned.
Figure 1
In 1988, the first CD-mediated enantioseparation in capillary gel electrophoresis (CGE) was demonstrated by Guttman et al.5 The physical incorporation of the cyclodextrin selector into a porous polyacrylamide gel matrix without covalent bonding for the enantioseparation of dansyl a-amino acids may be regarded as a hybrid of CEC.

Figure 2
Three modes of CEC may be distinguished (Figure 2).3
- The chiral selector is bonded to the inner surface of the open-tubular column: o-CEC.
- The chiral selector is bonded to a support, which is packed into the capillary: p-CEC.
- The chiral selector is bonded to a monolith4 (or rod) present in the capillary: rod-CEC.
o-CEC
In 1992, enantioselective o-CEC (also called OTCEC) was established by immobilizing Chirasil-β-Dex (a permethylated β-cyclodextrin bonded via a single octamethylene spacer to poly(dimethylsiloxane), which was originally developed for o-GC, [Figure 3(a)]) on the inner wall of a capillary [see Figure 2 (top) and Figure 4] and using the polymer-coated column for the enantioseparation of racemic mixtures added to the buffer system.6 The hyphenation of o-CEC and MS–MS was subsequently demonstrated for the enantiomers of hexobarbital of an extracted spiked urine sample at a concentration of 150 ng/mL.7 The o-CEC approach can be combined with the CEKC-mode1 by adding CMPAs to the buffer solution in the CD-coated capillary. Depending whether negatively or positively charged cyclodextrin derivatives are added to Chirasil-β-Dex, either compensation or enhancement of enantioselectivity is observed.8 In the case of compensation of enantioselectivity, the addition of increasing amounts of the anionic CD selector in the mobile phase leads first to peak coalescence and eventually to a reversal of the elution order of the enantiomers on the single o-CEC column coated with Chirasil-β-Dex.9 In a novel unified approach all contemporary chiral open-tubular chromatographic methods (i.e., o-GC, o-SFC, o-LC and o-CEC) could be realized for the enantioseparation of hexobarbital with a single capillary column (1 m x 0.05 mm i.d.) coated with 0.25 µm Chirasil-β-Dex, for example, thereby demonstrating the advantage of column miniaturization for o-GC also, which usually uses larger column formats (Figure 5).10 As expected, a higher efficiency is observed for o-CEC due to the plug-type flow profile compared with o-SFC and o-LC, which are governed by the parabolic flow profile.11 According to theory,10 peak broadening occurs in o-LC and o-CEC at increasing retention factors > 2 (the second peak in Figure 5). It was found that the number of racemates resolved by o-CEC is limited compared with enantioselective o-GC for reasons that are not yet known.
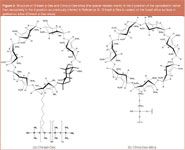
Figure 3
p-CEC
In packed capillary electrochromatography (p-CEC) enantioseparation is performed in capillaries filled with typical chiral HPLC materials. The packing bed is fixed by frits such as sintered silica [Figure 2 (middle)]. A serious drawback in p-CEC is the formation of bubbles caused by differences of the electroosmotic flow (EOF) at the interface between the frit and the unpacked part of the capillary or by Joule heating. This deleterious effect leads to baseline noise or even a breakdown of the current. To prevent air bubble formation, the separation can be performed under a slight overpressure (up to 12 bar) performed by pressurizing the inlet and the outlet buffer vials. Alternatively, an HPLC pump can be coupled to the inlet vial and the EOF is complemented by a pressure-driven flow. This hybrid form of pure p-CEC and packed capillary liquid chromatography (p-CLC) is called pressure-supported CEC, pressure-assisted CEC or electro-HPLC. The UV detection occurs in the empty part of the capillary. On-line coupling with MS represents a powerful tool because the chiral selector is covalently linked to the separation bed and can not interfere in the MS detection. In recent times, enantioseparations in non-aqueous CEC evolved as an attractive alternative because some chiral stationary phases (CSPs) perform better under non-aqueous conditions than under reversed-phase (RP) conditions. Usually the background electrolyte consists of methanol or acetonitrile containing salts such as ammonium acetate, triethylammonium acetate (TEAA) or 2-(N-morpholino)ethanesulphonic acid (MES).

Figure 4
The first approach of a packed capillary containing a cyclodextrin stationary phase for enantioseparation by p-CEC was described by Li and Lloyd.12 Enantioseparations of some neutral (hexobarbital, benzoin) and anionic (derivatized amino acids) compounds were observed on a β-cyclodextrin modified stationary phase obtained by emptying a Cyclobond I HPLC column (Actec, USA). The direction of the EOF could be controlled by variation of the background electrolyte. Lelièvre et al. used silica-linked hydroxypropyl-β-cyclodextrin as CSP for the enantioseparation of chlorthalidone and mianserin.13

Figure 5
Permethyl-β-cyclodextrin-derived stationary phases were applied by Wistuba and Schurig in two approaches:14,15 (i) the permethylated-β-cyclodextrin was covalently linked to silica via a thioether spacer [Chira-Dex-silica, Figure 3(b)], or (ii) the permethyl-β-cyclodextrin was bonded via a single octamethylene spacer to poly(dimethylsiloxane) [Chirasil-β-Dex, Figure 3(a)], which was subsequently immobilized onto silica (Chirasil-Dex-silica). Pressure-supported CEC was used for enantioseparation of barbituric acids, α-methyl-α-phenylsuccinimide, γ-phenyl-γ-butyrolactone, methylthiohydantoin-proline, methyl mandelate, 1-(2-naphthyl)-ethanol, glutetimide, mecoprop methyl, diclofop methyl and fenoxaprop ethyl. The connection of an HPLC pump to the electromigrative system enabled the switching between the p-CLC and the p-CEC mode of operation and allowed the direct comparison with one single capillary. Figure 6 demonstrates the influence of the pressure support on the enantioseparation of mephobarbital by CEC. On Chira-Dex-silica, the elution time is clearly dominated by a substantial contribution of the EOF. Comparing p-CLC at 10 bar with p-CEC at 10 bar and 20 kV an approximately 10 times longer elution time was found in the p-CLC mode. A pressure of 140 bar in the p-CLC mode is required to achieve the same elution time at a comparable enantioseparation factor a, whereby the efficiency N is reduced by a factor of three in the pressure-driven system in agreement with theory.14

Figure 6
Per-phenylcarbamoyl-β-cyclodextrin-bonded silica particles were also used as chiral stationary phase for pressure-assisted p-CEC and p-CLC by Zhou et al.16 and Lin et al.17 Several racemic a-amidophosphonates and a series of neutral and basic drugs were successfully resolved. As expected, higher efficiencies and enantioresolution factors were found in the p-CEC mode.
Both aqueous and non-aqueous conditions were applied for the enantioseparation of negatively charged dansyl-amino acids by p-CEC with capillaries packed with native β- and γ-cyclodextrin-bonded silica particles available as commercial HPLC packing materials Chira-β-Dex and Chira-γ-Dex (Merck KGaA, Darmstadt, Germany).18 It was observed that the addition of a small amount of water to the non-aqueous mobile phase enhanced the enantioselectivity but increased the elution time. Also the pH, the concentration of the electrolyte and the nature of the mobile phase strongly influenced the elution time, the efficiency and the enantioselectivity. It was found that the direction of the EOF depends on the type of electrolyte added.
The use of binary CSP systems in p-CEC consisting of two chiral selectors (i.e., cyclodextrins and crown ethers or cyclams) were described by Gong et al.19 A series of crown ether-capped and cyclam-capped 3-(2-O-β-cyclodextrin)-2-hydroxypropylsilyl silica particles were used for enantiomeric and isomeric separations of high selectivity. The inclusion of Ni2+ ions into the substituted cyclams and their sidearm ligands led to a positively charged stationary phase (i) providing extra electrostatic interactions with ionizable solutes and (ii) enhancing the dipolar interactions with some neutral polar analytes.
Complementary to the covalent bonding of chiral selectors, the dynamic coating or physical adsorption of cyclodextrins on a support represents a straightforward and time-saving preparation of cyclodextrin-modified CSPs for p-CEC. Thus hydroxy-β-cyclodextrin was dynamically coated on bare silica20 or on diol-silica.21 Ye et al. developed a strong anion-exchange stationary phase dynamically modified with sulphated -cyclodextrin and compared it with a physically adsorbed one.22 The superiority of p-CEC with a dynamically modified stationary phase over that with a physically adsorbed one was demonstrated with respect to the repeatability of the migration time.
Generally, a typical HPLC material used as CSP for p-CEC produces a low EOF because most of the silanol groups are modified or end-capped. Zhang and El Rassi developed a stationary phase containing a hydrophilic sublayer to which a chiral top layer of hydroxypropyl-β-cyclodextrin was immobilized.23 While the sulphonated sublayer was engineered to provide a relatively strong EOF, the top layer was then responsible for the enantiorecognition.
The hyphenation of enantioselective CEKC1 and MS represents a powerful tool for the analysis of complex samples. The enantioseparation is achieved by adding a CMPA (i.e., a chiral selector or a chiral surfactant) to the background electrolyte. Yet, a serious problem arises from the contamination of the ion source with the non-volatile CMPA. To overcome this problem, rather cumbersome partial-filling strategies or pressurized-flow-counterbalance methods1 have to be used. In the CEC methodology the on-line coupling with MS is straightforward as the chiral selector is immobilized at the packed or monolithic separation bed. Von Brocke et al. reported on the on-line coupling of pressure-assisted CEC to coordination ion spray-MS (CIS-MS) for the enantioseparation of barbiturates and chlorinated alkyl phenoxypropanoates on a capillary packed with permethylated β-cyclodextrin modified silica (Chira-Dex-silica) (Figure 7).24 In CIS-MS the introduction of charged metal ions such as silver (I), cobalt (II), copper (II) and lithium (I) to the sheath liquid allows the smooth ionization of analytes via coordination. This method is suitable for nonpolar or weakly polar substances, which have a poor sensitivity in the traditional electrospray ionization (ESI) mode. The advantages of p-CEC/CIS-MS are high selectivity, high sensitivity, mild ionization and novel fragmentation pattern.24
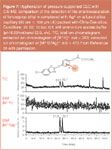
Figure 7
rod-CEC (monolithic approach)
To overcome the need of frits in p-CEC, the preparation of a continuous rod within the capillary by various monolithic approaches can be envisaged. Monolithic CSPs may consist either of organic polymers, inorganic silica networks or hybrids of both. Organic polymer monoliths are prepared by polymerization of organic monomers in the presence of a porogen and a crosslinker. The chiral selector is (i) copolymerized or (ii) grafted by polymerization. Silica-based monoliths are prepared by the sol-gel technology, whereas, the chiral selectors are immobilized by (i) covalent bonding, (ii) by physical adsorption or (iii) by encapsulation. Monoliths can also be prepared by particle-fixation techniques such as embedding the chiral modified silica particles into a sol gel matrix, into an organic polymer, by gluing it via a sol-gel process or by sintering unmodified silica particles at high temperatures followed by chiral derivatization.4 Three variants advanced in the present laboratory to prepare cyclodextrin-modified silica-based monoliths are depicted in Figure 8.

Figure 8
1. Cyclodextrin-modified silica-based monoliths
(i) Monoliths prepared by particle-fixation technique
Two different kinds of cyclodextrin-modified monoliths based on the particle-fixation techniques have been described: (i) bare silica particles packed into a capillary were covalently linked by sintering and the resulting monolith was subsequently modified with the cyclodextrin selector; or (ii) the cyclodextrin-modified silica particles packed into a capillary were glued together by the sol-gel technique.
Wistuba and Schurig prepared a robust and stable Chirasil-Dex monolith by sintering a packed bare silica bed inside the capillary after pre-treatment with sodium carbonate at 380 °C.25 Subsequently, the silica network was first coated with Chirasil-Dex and than thermally immobilized. The resulting Chirasil-Dex monolith (Variant I in Figure 8: the contact points between silica particles are clearly discernable) allows the application of a voltage up to 30 kV and a pressure in excess of 400 bar. The enantioseparation of several compounds such as barbituric acids, benzoin, methyl-α-phenylsuccinimide, MTH-proline, mecoprop methyl, fenoxaprop methyl, carprofen and ibuprofen by rod-CEC and pressure-supported rod-CEC was feasible. Comparison of rod-CEC and capillary liquid chromatography (rod-LC) using a single column in a unified experimental set-up showed that rod-CEC leads to an approximately two to three times higher efficiency. In rod-CEC the column efficiency was up to 88000 plates per metre. After a four month operation period of a single column, only minor variations in elution time and loss of resolution were observed.
A sol-gel-glued cyclodextrin-modified silica monolith was prepared by Wistuba and Schurig by packing first the capillary with permethyl-β-cyclodextrin modified silica particles (Chira-Dex-silica) and subsequently fusing the packing bed by an in situ sol-gel process.26 The resulting Chirasil-Dex monolith (Variant II in Figure 8) is stable toward voltage (30 kV) and pressure (300 bar), possesses a high efficiency (up to 100000 plates per metre) and is suitable for the enantioseparation of barbituric acids, thiopental, MTH-proline, methyl-α-phenylsuccinimide, chlorinated alkyl phenoxypropanoates, PCBs, carprofen and 2,2,2-trifluoro-(9-anthranyl-ethanol) (for examples see Figure 9). In the pressure-assisted rod-CEC mode the efficiency is twice as high as in the capillary liquid pressure mode. The scanning electron microscope (SEM) analysis of the monolith (see Figure 8, centre) shows, that the cyclodextrin-modified silica particles are fused and not embedded in a sol-gel matrix. A comparison with the Chirasil-Dex modified silica monolith prepared by the sol-gel process (Figure 8, right) reveals a quite different structure of the silica skeleton.

Figure 9
(ii) Monoliths prepared by the sol-gel technique
The sol-gel method based on the polycondensation of alkoxysilanes resulted in monolithic columns with lower flow resistance (high flow throughput) and good mechanical strength.
The first cyclodextrin-modified monolith (Variant III in Figure 8) fabricated in a two-step process was described by Kang et al. for the enantioseparation of mephobarbital, hexobarbital, benzoin and carprofen.27 First, a porous monolith was prepared inside the capillary by the sol-gel technique. After gelation a hydrothermal treatment at 100 °C was performed to prevent the sol-gel matrix from cracking during the drying process. Second Chirasil-Dex [Figure 3(a)] was coated and thermally immobilized to give a non-extractable CSP. Moreover, the amount of the porogen polyethylene glycol added had a pronounced effect on the pore size (Figure 10). A high column efficiency of about 92000 plates per metre was obtained for the first eluted enantiomer of the model analyte hexobarbital. Columns were stable with a good run-to-run reproducibility. Unfortunately, the column-to-column reproducibility was quite poor.
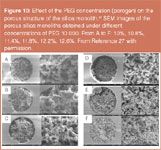
Figure 10
Also Chen et al. fabricated cyclodextrin-modified monoliths by the sol-gel process but in contrast to the above mentioned method the native β- and γ-cyclodextrin selectors were directly linked to the silica-network via a (3-isocyanatopropyl)triethoxysilane spacer.28 Several dansyl-amino acids were resolved with high column efficiency (49 000 plates per metre found for dansyl-threonine). The chiral monolithic column showed a good stability over a period of more than a hundred runs.
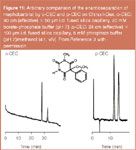
Figure 11
(iii) Comparison of various modes of cyclodextrin-modified CEC
The o-CEC and p-CEC/rod-CEC mode of operations use totally different capillary formats and any comparison is, therefore, arbitrary. Nevertheless, in Figure 11 the enantioseparation of mephobarbital on Chirasil-Dex by o-CEC and p-CEC is shown. While o-CEC produces a higher efficiency at a longer analysis time, p-CEC offers a shorter analysis time at a reduced efficiency. In Figure 12 a tentative comparison of all investigated cyclodextrin-modified modes of CEC (Figure 2) is attempted. In the p-CEC mode, a better resolution of PCB 132 is obtained with Chirasil-β-Dex-silica as compared with Chira-Dex-silica devoid of an apolar polysiloxane matrix. The advantages and disadvantages of the techniques are summarized in Figure 13. At present the state-of-the-art of enantioselective CEC employing Chirasil-Dex and Chira-Dex modified CSPs is still confined to the research area. Commercialization may in the future lead to wider applications in the realm of widespread chiral analyses (Figure 1).
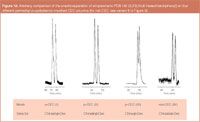
Figure 12
2. Cyclodextrin-modified organic polymeric monoliths
Synonyms for organic polymeric monoliths are continuous beds, rods or continuous polymeric rods. Two structurally quite different kinds of organic monoliths exist: (i) homogeneous gels and (ii) rigid porous polymeric monoliths. Homogeneous gels represent a visually transparent or only slightly opalescent separation media. The structure of rigid porous polymeric monoliths consists of small particles that are covalently linked and attached to the internal capillary wall. In principle, the homogenous gel is an almost ideal chromatographic support because of its high porosity and the fact that the Eddy diffusion is negligible. The polymer matrix of homogeneous gels consisting of low cross-linked polyacrylamide (PAA) is rather soft and highly swollen. Because of the high backpressure the mobile phase cannot be transported by a hydrodynamic flow but only by the EOF. It distinguishes itself from the quite similar gel used in capillary gel electrophoresis (CGE) by copolymerized functional ligands (stationary phase) and charged groups (for generation of EOF).4,29 The stationary phase of organic polymeric monoliths can be prepared in situ in the capillary or on a microfluidic chip by a single-step polymerization of organic monomers in the presence of a crosslinker and a porogen.

Figure 13
(i) Rigid polymeric monolith
Pumera et al. modified a neutral and a negatively-charged polyacrylate based monolith with either physically adsorbed or covalently bonded β-cyclodextrin derivatives.30 While no enantioselectivity was observed with both neutral and negatively charged monolith with physically absorbed tert.butyl-β-cyclodextrin, racemic ibuprofen could be resolved using a negatively charged monolith with a chemically linked β-cyclodextrin derivative. The negative charge of the monolith was produced by the co-monomer 2-acrylamido-2-methyl-1-propanesulphonic acid. The chiral selector was introduced by copolymerization of peracetyl-2'-O-allyl-β-cyclodextrin. The enantioseparation of ibuprofen by the two methods rod-CEC and normal HPLC was compared. It was found that the resolution factor observed by HPLC was somewhat higher than by rod-CEC (RS(CEC) = 2.45; RS(HPLC) = 2.97) but the theoretical plate number per metre for the first eluted enantiomer was found to be lower (NCEC = 1810; NHPLC = 380).
A claimed polyrotaxane-modified monolith prepared in a one-step in situ synthesis and its use in CEC was described by Kornyšova et al.31 Rotaxanes consist of a reactive axial moiety and an inert macrocyclic moiety, which are mechanically and irreversibly linked in a supramolecular structure. Upon polymerization, a gel forms with immobilized cyclic molecules. Cationic or anionic cyclodextrins were co-polymerized with neutral acrylic-type monomers, while neutral cyclodextrin needed vinylsulphonic acid as an ionic co-monomer. The enantioseparation of ibuprofen and metoprolol with neutral -cyclodextrin was observed even though the polyrotaxane structure was not unambiguously proved.
(ii) Cyclodextrin-modified homogeneous gels
The first generation of cyclodextrin-modified homogeneous gels used as CPSs for rod-CEC was described by Koide and Ueno.32 A negatively and positively charged polyacrylamide-based homogenous gel with incorporated poly β-cyclodextrin or poly carboxymethyl-β-cyclodextrin was used for enantioseparation of several cationic, neutral and anionic compounds. 2-Acrylamido-2-methylpropanesulphonic acid (AMPS) was used as a negatively charged, and N-(2-acrylamidoethyl)triethylammonium iodide as a positively charged monomer, respectively. Incorporation of monomeric β-cyclodextrin or carboxymethyl β-cyclodextrin was unsuccessful because the cyclodextrin was eluted from the capillary by the EOF. Thus, negatively and positively charged polyacrylamide gels with covalently linked allyl carbamoylated β-cyclodextrin as monolithic stationary phase were developed.33 These monolithic phases allow high efficient enantioseparations with enantioresolution factors RS up to 7.1. The positively charged cyclodextrin-functionalized monolith was tailored for anionic and neutral compounds, the negatively charged one for neutral and cationic compounds (Figure 14). In some cases an improvement of enantioselectivity was achieved by the addition of a chiral crown ether. The stability and the within-run and between-run reproducibility of these chiral modified monoliths were satisfactory.

Figure 14
Végvári et al. reported on very similar negatively and positively charged polyacrylamide (PAA) gels copolymerized with 2-hydroxy-3-allyloxy-propyl-β-cyclodextrin.34 High theoretical plates per metre (up to 570 000) and enantioresolution factors RS (up to 8.18) were observed for the enantioseparation of several acidic, neutral and basic drugs. These homogeneous continuous beds were easily prepared and were stable for more than one month.
A new development in this field is the use of microchip monolithic arrays for enantioselective rod-CEC. Zeng et al. reported on a PDMS microchip β- or γ-cyclodextrin-modified PAA gel monolithic column for the enantioseparation of FITC-labelled amino acids and FITC-labelled dansyl-amino acids. Using LIF-detection, resolution factors RS up to 2.53 were achieved.35 In this interesting approach, cyclodextrin acts not only as a chiral selector but also as a crosslinker because of its allyl-modified spacer.
Further Information
A review recently published by Schmid and Gübitz may be consulted [G.Gübitz and M.G. Schmid, J. Chromatography A, 1204, 140–156 (2008)].
Dorothee Wistuba received her PhD from the University of Tübingen in 1986 in Organic Chemistry. She has been a senior scientist in the field of chromatography and capillary electromigration methods for more than 20 years at the Institute of Organic Chemistry, Tübingen, Germany. Volker Schurig is professor of organic chemistry, stereochemistry and separation techniques at the University of Tübingen, Germany. His work encompasses the separation of enantiomers by complexation and inclusion GC as well as various electromigration methods. Together with H.-P. Nowotny, he used modified cyclodextrins in polysiloxane matrices as versatile chiral selectors in high-resolution capillary columns. His applications of chiral analyses encompass chirality pool synthesie, asymmetric catalyses, kinetic resolutions, enzymatic transformations, pheromones, flavours, fragrances, organochlorines, inhalational anesthetics and pharmaceuticals. Reaction chromatography is another topic of interest. He published 380 scientific papers and lectured at 250 national and international venues. (homepage: www.uni-tuebingen.de/schurig)
References:
1. B. Chankvetadze, Capillary Electrophoresis in Chiral Analysis, Wiley, Chichester, 1997 and J. Chromatogr. A, 1168, 45–70 (2007).
2. D. Wistuba and V. Schurig, Electrophoresis, 21, 4136–4158 (2000).
3. V. Schurig and D. Wistuba, Electrophoresis, 20, 2313–2328 (1999).
4. D. Wistuba and V. Schurig, J. Sep. Sci., 29, 1344–1352 (2006).
5. A. Guttman et al., J. Chromatogr., 448, 41–53 (1988).
6. S. Mayer and V. Schurig, J. High Resolut. Chromatogr., 15, 129–131 (1992) and J. Liq. Chromatogr., 16, 915–931 (1993). European Patent EP 0 610 419 B1 from 24. 5. 1995.
7. V. Schurig and S. Mayer, J. Biochem. Biophys. Methods, 48, 117–141 (2001).
8. H. Jakubetz, M. Juza and V. Schurig, Electrophoresis, 18, 897–904 (1997) and 19, 738–744 (1998).
9. S. Mayer, M. Schleimer and V. Schurig, J. Microcol. Sep., 6, 43–48 (1994).
10. V. Schurig et al., J. Chromatogr. A, 694, 119–128 (1995).
11. H. Jakubetz, H. Czesla and V. Schurig, J. Microcol. Sep., 9, 421–431 (1997).
12. S. Li and D.K. Lloyd, J. Chromatogr., 666, 32–335 (1994).
13. F. Lelièvre et al., J. Chromatogr. A, 723, 145–156 (1996).
14. D.Wistuba et al., J. Chromatogr. A, 815, 183–188 (1998).
15. D.Wistuba and V. Schurig, Electrophoresis, 20, 2772–2778 (1999).
16. A. Zhou et al., Anal. Chim. Acta, 547, 158–164 (2005).
17. B. Lin et al., Electrophoresis, 27, 3057–3065 (2006).
18. D. Wistuba, K. Cabrera and V. Schurig, Electrophoresis, 22, 2600–2605 (2001).
19. Y. Gong et al., J. Heterocyclic Chem., 38, 1317–1321 (2001), Tetrahedron Lett., 43, 2463–2466 (2002) and Anal. Chem., 75, 1348–1354 (2003).
20. W. Wei et al., J. Microcol. Sep., 11, 263–269 (1999).
21. M. Zhang and Z. El Rassi, Electrophoresis, 21, 3126–3134 (2000).
22. M. Ye et al., Electrophoresis, 22, 518–525 (2001).
23. M. Zhang and Z. El Rassi, Electrophoresis, 21, 3135–3149 (2000).
24. A. von Brocke et al., Electrophoresis, 23, 2963–2972 (2002).
25. D. Wistuba and V.Schurig, Electrophoresis, 21, 3152–3159 (2000).
26. D. Wistuba, L. Banspach and V. Schurig, Electrophoresis, 26, 2019–2026 (2005).
27. J. Kang, D. Wistuba and V. Schurig, Electrophoresis, 23, 1116–1120 (2002).
28. Z. Chen et al., Electrophoresis, 24, 2550–2558 (2003).
29. A. Végvári, J. Chromatogr. A, 1079, 50–58 (2005).
30. M. Pumera et al., J. Liq. Chromatogr. Rel. Techn., 25, 2473–2484 (2002).
31. O. Kornyšova et al., J. Chromatogr. A, 971, 225–235 (2002) and Biochem. Biophys. Methods, 50, 217–232 (2002).
32. T. Koide and K. Ueno, Anal. Sci., 16, 1065-1070 (2000).
33. T. Koide and K. Ueno, J. High Resol. Chromatogr., 23, 59–66 (2000) and J. Chromatogr. A, 909, 305–315 (2001).
34. A.Végvári et al., Electrophoresis, 21, 3116–3125 (2000).
35. H.-L. Zeng et al., Anal. Chim. Acta, 551, 1–8 (2005), Talanta, 69, 226–231 (2006) and J. Capillary Electrophoresis and Microchip Tech., 10, 19–24 (2007).
Best of the Week: Food Analysis, Chemical Migration in Plastic Bottles, STEM Researcher of the Year
December 20th 2024Top articles published this week include the launch of our “From Lab to Table” content series, a Q&A interview about using liquid chromatography–high-resolution mass spectrometry (LC–HRMS) to assess chemical hazards in plastic bottles, and a piece recognizing Brett Paull for being named Tasmanian STEM Researcher of the Year.
Using LC-MS/MS to Measure Testosterone in Dried Blood Spots
December 19th 2024Testosterone measurements are typically performed using serum or plasma, but this presents several logistical challenges, especially for sample collection, storage, and transport. In a recently published article, Yehudah Gruenstein of the University of Miami explored key insights gained from dried blood spot assay validation for testosterone measurement.
Determination of Pharmaceuticals by Capillary HPLC-MS/MS (Dec 2024)
December 19th 2024This application note demonstrates the use of a compact portable capillary liquid chromatograph, the Axcend Focus LC, coupled to an Agilent Ultivo triple quadrupole mass spectrometer for quantitative analysis of pharmaceutical drugs in model aqueous samples.