The Role of Selectivity in Extractions: A Case Study
LCGC North America
The selective removal of a fat substitute in food products is discussed to demonstrate options for obtaining selectivity during extraction.
Many of the extraction techniques developed over the past generation tout selectivity among their advantages. In reality, solvent selection and the use of stationary (sorbent) phases are the main mechanisms for providing selectivity. Therefore, selectivity is often limited to isolation of classes of compounds rather than individual structures. In this column installment, the selective removal of a fat substitute in food products is discussed to demonstrate options for obtaining selectivity during extraction.
Over the past generation or so, myriad extraction techniques were developed that have generally improved yields, lessened the amount of organic solvent used, and minimized time. Additionally, many of these techniques claim advantages concerning selectivity.
Selectivity is the ability to determine the analytes of interest in preference to other sample components (potential interferents). A recent installment of this column (1) advocated that selectivity can stem from any point in the analytical process, but as a general rule, selectivity arises from separations, selective detection schemes, and selective chemical reactions. These approaches can balance each other. For example, if an analytical separation is not completely sufficient, the use of a selective detection method like mass spectrometry (MS) or fluorescence spectroscopy can offer the balance of the required selectivity provided that the unseparated components do not suppress the detector signal.
Majors described "just enough" sample preparation (2) in which method selectivity is matched to the qualitative or quantitative analytical requirements. For example, the QuEChERS (quick, easy, cheap, effective, rugged, and safe) method for extracting pesticides from fruits and vegetables combines salting out partitioning with dispersive solid-phase extraction (SPE) to remove matrix components, allowing effective chromatography and MS detection. As Majors points out and illustrates in Figure 1 from his original column, increasing complexity in an analytical procedure typically leads to greater selectivity.
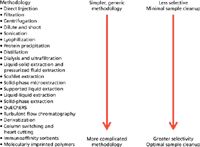
Figure 1: Just-enough sample preparation represents a continuum of methodologies.
Turning our attention back to modern extraction methods, the fundamental driving force of the technique leads to the element of selectivity. A number of sorbent-based methods, such as SPE, solid-phase microextraction, and stir-bar sorbent extraction, use chromatographic stationary phases to isolate solutes of interest from gaseous or liquid samples. Analytes are retained by their attraction to a stationary phase of similar polarity and are selectively eluted via choice of an appropriate solvent. The techniques aimed at solid samples, including supercritical fluid extraction (SFE), pressurized fluid extraction, microwave extraction, and ultrasound extraction, rely on the application of energy (often heat) to drive the analyte into an appropriate solvent. In all of these techniques, both sorbent- and solvent-based, the key to selectivity is the match between analyte polarity and polarity of the extracting phase. In other words, "like dissolves like." Thus, extractions are usually considered crude separation techniques, providing compound class selectivity and less utility for the selective isolation of specific, individual compounds. Of course, volatility is the major contributor to selectivity for gas-phase techniques.
If the primary selectivity mechanism in extractions is solute polarity (that is, matching solute polarity with the solvent or sorbent following the "like dissolves like" principle), is selectivity possible during chemical extraction? Is selectivity beyond compound class selectivity possible? Do extractions need to be selective or is selectivity solely a function of subsequent chromatography and detection?
To look at an example of extraction selectivity within the "like dissolves like" polarity context, let's consider the example of fat analysis in food products and, more specifically, the example of sucrose ester fat substitutes.
Fatty Acid Methyl Ester Analysis
The United States Nutrition Labeling and Education Act (NLEA) of 1990 requires the labeling of selected nutrients on prepackaged food products. One issue with this requirement deals with the concept of "total fat." What is a "fat"? Are lipoproteins considered lipid or protein? The next concern is their analysis. If "fats" are based on the fatty acid moiety, how can they be measured? Fatty acids are not volatile enough for gas chromatography (GC) analysis. They do not contain any chromophores necessary for ultraviolet detection in liquid chromatography (LC). (Remember, at the time, LC–MS was not as widely accepted as it is currently.) The polarity of the acidic group can irreversibly adsorb to active sites on chromatographic stationary phases via hydrogen bonding, depending on the type of chromatography. Consequently, the total fat listed on nutritional labels is based on the acid hydrolysis and formation of methyl esters of an organic extract, using a nonpolar solvent, of the food product. The "total fat" listed on the nutritional label is that of a triglyceride based on the resulting fatty acid methyl ester (FAME) composition (3,4). An overview of the formation of methyl esters from triglycerides is presented in Figure 2.

Figure 2: Triglycerides are hydrolyzed and esterified with methanol to form FAME. The R groups of the triglyceride are typically fatty acids with a carbon chain length of 14â24 and up to two double bonds.
In the FAME method, samples are dissolved in a nonpolar solvent and a catalyst like BF3 dissolved in methanol is added. Sometimes methanolic acid or base is used. After mild heating, back-extraction with water removes the polar components. The FAME sample is dried and characterized by GC with flame ionization detection (FID). The esterification is facilitated with an alkylation derivatizing agent to condense the carboxyl group of the fatty acid with the methanol hydroxyl. The catalyst aids the reaction by protonating the acid group to promote the formation of the ester and water. The stability of the methyl ester, or FAME, allows GC separation by boiling point or unsaturation.
The Procter and Gamble Company began marketing sucrose esters, called olestra or the tradename Olean, as fat substitutes in the mid-1990s. The sucrose ester structure is shown in Figure 3. In this figure, the R group is either hydrogen or any fatty acid. By varying the number of fatty acids connected to the sucrose molecule by ester linkages or by changing the carbon chain length of the fatty acids, the properties of the olestra molecule can be altered. Under appropriate conditions, the olestra molecule can have boiling points, viscosity, mouth feel, and other properties similar to common vegetable oils. Because they are not naturally occurring lipids (though they are made from naturally occurring compounds), the sucrose esters are not subject to enzymatic digestive action. Hence, they can be substituted for vegetable oils in selected applications, such as the frying of potato chips and similar salted snacks.

Figure 3: Structure of sucrose ester (olestra) fat substitutes created from sucrose esterified with six to eight fatty acids.
If we review the acid hydrolysis and esterification reactions for the FAME analysis, olestra in food products would be hydrolyzed along with triglycerides and other fats. The resulting FAMEs would be indistinguishable regarding their source, olestra or triglyceride. Thus, a selective analysis to determine NLEA "total fats" in the presence of olestra is needed. Here we will present three possibilities to garner the necessary selectivity during the sample preparation process.
Supercritical Fluid Extraction
Perhaps the easiest method, conceptually, to address the isolation of FAME from total fats from those originating from olestra would be at the level of the extraction, meaning we would selectively extract olestra from the total fats. (That is, we're assuming that we must perform FAME analysis of total fats to comply with the requirements of the NLEA.) This brings us back to the issue of solvent polarity or "like dissolves like." Because olestra is designed to have properties substantially similar to vegetable oils, which are composed primarily of triglycerides, the solvents used in the dissolution and extraction of either olestra or triglycerides would likely be very similar. This brings us to the solvent extraction method where we have the most variation in solubility conditions with a single solvent: SFE. SFE almost always uses carbon dioxide, perhaps mixed with small amounts of organic cosolvents, near or above its critical point of 31.1 °C and 72.9 atm. Lipids and lipophilic materials are highly soluble in supercritical carbon dioxide, and the use of this solvent for the extraction and fractionation of lipids is well-reviewed (5–7). By making subtle changes in the operating temperature or pressure, somewhat dramatic changes in solvating ability can occur. These changes may allow the fractionation of members of a single compound class on the basis of either polarity or molecular weight. Because the molecular weights of olestra molecules are at least double that of triglycerides, it is conceivable that SFE could be used to either selectively extract olestra and triglycerides from each other, or to selectively precipitate one from the other. This has not been reported in the peer-reviewed literature, so will remain theoretical for now. No other solvent extraction methods will be able to achieve this level of selectivity as easily.
Solid-Phase Extraction
The next step up in complexity toward gaining the requisite selectivity during the sample preparation of olestra-containing food products would be to use a sorbent-based method to separate olestra from total fats postextraction. SPE is the most basic of these techniques and perhaps the most directly applicable to our hypothetical scenario. SPE can, in many ways, be regarded as an elementary form of LC. A stationary phase is placed onto a support material and put into a cartridge, disk, or other vehicle. Liquid samples are placed onto the SPE sorbent where total retention is achieved. Then analytes and interferents are isolated from each other by the judicious elution with selective solvents.
Tallmadge and Lin (8) used reversed-phase LC to determine the percent olestra in lipid samples. They found an octadecylsilane column (Zorbax, Agilent Technologies) appropriate to separate olestra from other lipophilic sample components in samples of soybean-oil olestra and heated or unheated cottonseed-oil olestra in soybean oil. The percentage of olestra in these samples varied from 5% to 90% and relative recoveries of 99.2% to 106.0% were reported. Thus, it seems possible that with minimal additional method development a protocol could be developed that involves an extraction of total fats and olestra from the sample food product, SPE separation of olestra from the total fats, and FAME analysis of the total fats.
Lipase Hydrolysis
Simultaneously, perhaps the most obvious and the most direct means of addressing the proposed situation is to explore the fundamental chemistry behind the problem. Again, olestra is created by esterification of sucrose with fatty acids, but because it lacks the glycerol backbone, it is not subject to enzymatic digestion as are triglycerides. Can this resistance to digestion be exploited in the conversion of total fats to FAMEs to the exclusion of olestra? This is the approach taken in a method validated under the Association of Official Analytical Chemists (AOAC) Peer-Verified Methods Program (9). A modified version of AOAC Method 983.2.3 was used, in which a chloroform–methanol extraction of olestra-containing snacks was performed. This extract contained both the total fat and olestra. The hydrolysis portion of the FAME analysis used a lipase to hydrolyze the total fats, leaving the unaltered olestra. The fatty acids resulting from the lipase hydrolysis were precipitated as calcium salts and the olestra was extracted with hexane. The fatty acid salts were redissolved and esterified before GC analysis. Recoveries of 101% (6% relative standard deviation [RSD]) for total fat and 104% (6% RSD) for saturated fat were reported. Repeatability and reproducibility were also studied and the method was standardized for fatty acid carbon chains of 6–24. This represents the official method for the determination of total fats in packaged food products containing olestra fat substitute.
Summary
Analytical selectivity can occur during any step of an analytical method, but typically it occurs during the separation or detection steps rather than during sample preparation. Selectivity during chemical (analytical) extractions is almost exclusively limited to solute polarity. Consequently, selective extractions beyond compound class separations will be difficult. Although selective sample preparation is not the typical case, increasing complexity of the procedures can lead to selective analysis. This column installment presented a scenario in which selectivity during the characterization procedure was not possible, but selectivity during solvent extraction (SFE) and sorbent-based extraction (SPE) or via selective reactions was shown.
References
(1) R. Majors and D. Turner, LCGC North Am. 30(2), 100–110 (2012).
(2) R. Majors, LCGC North Am. 30(12), 1024–1031 (2012).
(3) AOCS Method Ce 1-62, "Fatty Acid Composition by Gas Chromatography," American Oil Chemists Society Official Methods (2005).
(4) AOAC Method 996.06, "Fat (Total, Saturated, and Unsaturated) in Foods," 18th edition Association of Official Analytical Chemists Methods.
(5) J. Martinez and A.C. deAguiar, Curr. Anal. Chem. 10, 67–77 (2014).
(6) F. Sahena, I.S.M. Zaidul, S. Jinap, A.A. Karim, K.A. Abbas, N.A.N. Norulaini, and A.K.M. Omar, J. Food Eng. 95, 240–253 (2009).
(7) F. Temelli, J. Supercrit. Fluids 47, 583 (2009).
(8) D.H. Tallmadge and P.Y. Lin, J. AOAC Intl. 76, 1396–1400 (1993).
(9) D. Schul, D. Tallmadge, D. Burress, D. Ewald, B. Berger, and D. Henry, J. AOAC Intl. 81, 848–849 (1998).
Douglas Raynie is an Associate Research Professor at South Dakota State University. His research interests include green chemistry, alternative solvents, sample preparation, high resolution chromatography, and bioprocessing in supercritical fluids. He earned his PhD in 1990 at Brigham Young University under the direction of Milton L. Lee.

Douglas Raynie
Ronald E. Majors "Sample Prep Perspectives" Editor Ronald E. Majors is an analytical consultant and is a member of LCGC's editorial advisory board. Direct correspondence about this column to "Sample Prep Perspectives," LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, Suite 210, Iselin, NJ 08830, e-mail lcgcedit@lcgcmag.com.

Ronald E. Majors

Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)





