The Future of Miniature Mass Spectrometers and a Path Forward: A Few Thoughts from an Academic Researcher
LCGC North America
A discussion of the future role of miniature MS systems, the need for simplification in operation, the role of ambient ionization, and challenges in development and commercialization.
Mass spectrometry (MS) serves as a versatile and effective tool in chemical analysis. It provides highly specific molecular information at excellent sensitivity. The emerging miniature MS systems could potentially be used outside analytical laboratories by personnel not trained in analytical chemistry, and thereby the range of applications for MS could also be significantly broadened. This column installment defines the future role of miniature MS systems in specialized analysis, justifies the need for simplification in operation, proposes a development approach involving ambient ionization, and delineates challenges in development and commercialization.
When we mention miniature mass spectrometers, it often brings to mind handheld research prototypes such as the Mini 10 or Mini 11 systems developed at Purdue University (West Lafayette, Indiana) or commercial products that specialize in homeland security applications (1). A marked advance in the same category is the recent development at Purdue of a backpack mass spectrometer that has a sampling probe that can scan ground surfaces for in-field detection of explosives (2). In this column installment, however, we contemplate a different type of miniature mass spectrometry (MS) analysis systems, such as the Mini 12 system (3). Also developed at Purdue, the Mini 12 system weighs 25 kg, is as compact as a desktop computer, and could prove useful in the field of biomedicine as well in the pharmaceutical, chemical, and agrochemical industries. A primary motivation for developing such a system is to enable physicians, nurses, and biologists to analyze samples at their desks, obviating the need to send the samples to an analytical laboratory.
Miniature Mass Spectrometry Analysis System Defined
In the past, the term miniature mass spectrometer has been used for a variety of devices that fall within a broad range of system completeness or self-sustainability. The miniature mass analyzers or vacuum manifold assemblies by themselves have all been called miniature mass spectrometers previously. Finally, complete instrument packages were developed to perform vacuum pumping, ionization, mass analysis, instrument control, and data acquisition. As demonstrated by the Mini 11, a mass spectrometer, even with multistage MS-MS capability, can be made to weigh only 4 kg (4). Such a miniaturized instrument by itself, however, would not be practically useful because it could not perform complete chemical analyses starting from raw samples (1). As for a mobile chemical analysis laboratory, additional equipment for sample preparation and chromatographic separation is always needed and could require more space than the mass spectrometer. Thus, sample preparation before MS analysis must also be done using miniaturized equipment and highly autonomous procedures.
Assuming such miniaturization is feasible at a system level, a biologist doing a preclinical study could almost effortlessly perform routine work such as finding the concentration of a drug metabolite in blood. To do that, he or she would draw about 0.5 μL of blood from a study animal (for example, a mouse), drop the blood onto a paper substrate inside a disposable sample cartridge, push the cartridge into the analysis system, and then wait 60 s for a report of the concentration. With such a small amount of sample required, this type of analysis would be minimally invasive. More importantly, the biologist would not need to program the instrument, telling it what to look for or what to do. Instead, a bar code on the cartridge would be scanned, and a presaved scan function would be automatically loaded and executed. The biologist also would not need to be concerned about the accuracy of measuring a 0.5-μL sample, because he or she would use a precut capillary, taking the blood by capillary action. The biologist could also expect a high degree of precision for the quantitation result because the internal standard (IS) would be precoated on the inside wall of the capillary (5), and the concentration calculated according to the analyte/IS ratio measured and a presaved calibration curve.
A similar system could be used by a nurse performing a blood test to identify a smoker (6), by a physician who must perform therapeutic drug monitoring to prescribe the correct dosage of cancer or immunosuppressive drugs (7), or by a police officer or anxious parent who wants to determine the presence or absence of illicit drugs in urine (8). To do these things, complete systems much smaller than the current systems used in analytical laboratories must be developed. Nevertheless, though we could never overemphasize the importance of a system's operational simplicity, we might indeed overemphasize the importance of its small size. Yet we must avoid doing so. If we can accommodate printers or copiers of various sizes, it might be ok for us to accept MS systems of 50 kg, as long as they can be operated like a printer or copier and fit unobtrusively in our offices.
Development Strategy
The focus of miniaturization used to apply mainly to instrumentation. However, the development of applications for small instruments such as the Mini 11 revealed that beyond the mass spectrometer itself, much remained to be addressed before a complete solution for chemical analysis could be offered outside the laboratory. Mass spectrometers are always at the end of the food chain and they just don't take raw stuff very well! The Mini 12, as a proof-of-concept prototype, was developed for exploring a solution at the system level.
The incremental approach to reducing equipment size (for instance, by adopting microextraction or microfluidic technologies for traditional sample preparation and chromatographic separation) might eventually deliver some good integrated solutions. However, direct MS analysis using ambient ionization has certainly already shown its potential (9–11). The term ambient ionization, coined by Professor R. Graham Cooks at Purdue, originally referred to a class of sampling ionization technologies for direct ionization of chemicals from samples in their raw or unprocessed "ambient" state (12). I often wonder whether by now Graham has regretted using this term, for so many researchers misconstrue ambient ionization as meaning ambient pressure ionization and therefore atmospheric pressure ionization, which refers to electrospray ionization (ESI) or atmospheric pressure chemical ionization (APCI). Both ESI and APCI are used under atmospheric pressure, but traditionally only with compounds extensively purified following sample preparation.
The potential of ambient ionization was originally demonstrated with desorption electrospray ionization (DESI) and direct analysis in real time (DART) (13), not to mention another 30-plus ambient ionization methods developed thereafter (9–11). Remarkable limits of detection have been achieved using direct sampling analysis without any chromatographic separations. Examples include the detection of chemical warfare agents at low-parts-per-billion (ppb) levels using DART (14), 0.62 pg/mL nicotine in gas-phase samples using extractive electrospray ionization (EESI) (15), 100 fmol of peptides using electrospray-assisted laser desorption ionization (ELDI) (16), and 0.2–40 ng amounts of drug molecules in plasma using DESI (17).
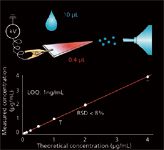
Figure 1: Paper spray ionization and direct quantitative analysis of imatinib in blood. Adapted from reference 7.
A promising strategy for the future development of miniature MS analysis systems, as the Mini 12 system has demonstrated, would be to combine ambient ionization methods with miniature mass spectrometers (18). In the Mini 12 system, paper spray ionization (7) (Figure 1) is used with a miniature ion-trap mass spectrometer. A blood sample is deposited on the triangle paper substrate inside a sample cartridge, forming a dried blood spot. After it is pushed into the system, about 10–30 μL of organic solvent is automatically added to the cartridge, and a voltage of about 4 kV is applied. The solvent elutes the organic compounds, such as drugs and their metabolites, and spray ionization occurs at the tip of the paper substrate. Two MS-MS scans are automatically performed on the analyte and its internal standard, which can be mixed in the sample by using an IS-coated capillary (5) or IS-printed paper substrate (19). As an example of the quantitation performance possible using the Mini 12, consider the analysis of amitriptyline in blood (3), which returned an limit of detection (LOD) of 7.5 ng/mL and a relative standard deviation (RSD) better than 10% (Figure 2).

Figure 2: Plot showing the performance of the Mini 12 MS system for the quantitation of therapeutic drugs in a blood sample. Adapted from reference 3.
Challenges and Solutions
The challenges associated with bringing miniaturized MS analysis systems to end users are quite considerable. The instrumentation and application must be researched and developed much further, and a good strategy will eventually be needed to develop the initial market for the products. Before we even begin to address those challenges, we must overcome a psychological barrier. MS has established itself as the "gold standard" for chemical analysis, and it is proudly announced as the most sensitive and specific technique for "general purpose" analysis. The development and continuous refinement of conventional, general-purpose MS analysis systems has, in turn, led to better resolution, mass accuracy, and wider dynamic ranges for both mass-to-charge ratio (m/z) and concentration. MS systems meeting with these criteria are therefore highly effective for analyzing a plethora of chemical and biological compounds over wide ranges of concentration and molecular weight. For example, Figure 3 shows a subset of chemical and biological compounds, ranging in mass from several hundred daltons to 16 kDa, in human blood that includes therapeutic drugs, amino acids, lipids, and proteins. In concentration, these compounds vary by more than nine orders of magnitude. Following delicate sample preparation and chromatographic separation, they can all be quantitatively analyzed using modern, commercial mass spectrometers. Indeed, the success of mass spectrometers has produced the high standard by which we judge their performance and guide instrument development. However, any attempt to transfer the capability of conventional mass spectrometers to miniature MS systems would sink the ship before the journey starts. To gain the convenience of using small systems, we must forgo something; we must compromise, and we must do so in a significant way.
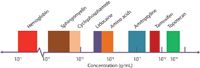
Figure 3: Exemplary chemical and biological compounds in human blood.
Can we make small MS systems with each specialized only for one compound from one sample? In such a case, we need to worry about only a narrow concentration range, a narrow m/z range (though perhaps not for hemoglobin — at least not yet), a single SRM (MS-MS) scan, and a single calibration curve. The chance for packaging these functions into a unit operated with minimal human intervention would be significantly larger. One must question whether manufacturers would profit by systems of restricted application range, but which would, nonetheless, reflect significant development and production costs. At the moment, we might have to blindly believe that the high-volume sale of units and their associated consumables, simply because of the convenience of use, would take care of the profit. This would make it critical to identify killer applications for launching the product.
Here I am depicting a future of MS with two distinct paths: one for the current commercial MS systems further advanced for discovery and research work and the other for the specialized and turnkey miniature MS systems developed for routine analysis.
Some technical challenges have already been identified. They include efficient extraction of target compounds from the complex sample; efficient transfer of the ions into the miniature mass spectrometer; and adequate performance for compound identification, in light of "compromised" instrument capability. Rapid development in the field of ambient ionization offers us hope that miniature MS systems using consumable sample cartridges would perform with adequate sensitivity.
A critical development in operation procedure would be the accurate transfer of small amounts of samples and the incorporation of internal standards for quantitation. The practical challenge lies in the associated procedures, which must be simple enough for users who are untrained in analytical techniques (5).The interface for coupling an ambient ionization source with a miniature mass spectrometer is another challenge. For instruments fitted with small pumps, ion transfer from air to mass analyzer has proved difficult. Currently, the only method developed is the discontinuous atmospheric pressure interface (20) used in the Mini 12 (3) and its predecessors, the Mini 10 (20) and Mini 11 (4). This is one area in the instrument development that particularly needs some major effort. People are also generally nervous about the mass accuracy and mass resolution of the miniature mass spectrometer, which no doubt would be somewhat compromised to achieve smaller size, lower weight, and lower cost of the system. Before we put enormous effort into tackling this problem, we should ask this question: How much could we tolerate the mass shifts and overlap of the isobaric peaks if MS-MS transitions are used for compound identification? Formulating a general answer would be difficult right now, and we probably should not look for a general solution in the future for miniature MS systems either. For a special package with a sampling ionization method optimized for target analytes, the specificity and reliability based on MS-MS can easily be tested. On-cartridge, real-time reactions can also be incorporated, improving the specificity based on the chemical structures of the target compounds (19).
The Path Forward in Research and Commercialization
The miniaturization of mass spectrometers used to be solely for instrumentalists. Now, however, the future development of complete analysis systems requires a major contribution by analytical chemists who possess extensive knowledge and experience in sample treatment and chromatography. We will need to persuade many of them to shift their interest from liquid chromatography (LC) columns to sample cartridges with integrated functions for real-time extraction and sampling ionization.

Dedication to R. Graham Cooks: An "Acorn" in Mass Spectrometry
Developing miniature MS analysis systems requires a comprehensive engineering capability for research and development, certainly a stretch for academic, analytical chemistry research groups, which have historically made major efforts to develop instrumentation for chemical analysis. In the past, the Jonathan Amy Facility for Chemical Instrumentation (JAFCI) at Purdue has served as an effective model for enabling the engineering capability to analytical chemists. In fact, the JAFCI model has been adopted by other chemistry departments nationwide. Current economic constraints, however, make establishing new facilities or even maintaining current ones difficult. Searching for alternatives, some analytical chemistry divisions have exploited their intrinsic connections with academic engineering departments such as chemical engineering and biomedical engineering. Some new initiatives nationwide among these departments suggest that their faculty's holding positions in both chemistry and engineering departments might be a sustainable way of creating and maintaining multidisciplinary research environments for developing chemical instrumentation. Such a setup would also provide an opportunity for engineering students and researchers to play a more active role, versus a supportive one in the JAFCI model, in the research and development of chemical instrumentation; as long as we tell them we are now stepping into the era of "MS sensors" and that Rapid Communications in Mass Spectrometry (RCM), International Journal of Molecular Sciences (IJMS), Journal of Mass Spectrometry (JMS), or Journal of The American Society for Mass Spectrometry (JASMS) are actually all engineering journals.
The future of the miniature MS analysis systems could be very bright; the path for their commercialization, however, could still be very difficult. It is not a natural move for any of the current major instrument companies to initiate the production of small systems. No doubt they all retain top-quality instrumentation scientists who can produce products of novel capabilities; however, it can be mentally torching to ask the builders of Mercedes-Benz automobiles to shift their interests to making scooters. Besides, market research plays such an important role nowadays in deciding which products to develop, and no valid data would be available for anything truly groundbreaking. Unless a "dictator" with a vision like Steve Jobs appeared in one of the large instrument corporations, development of miniature MS products would more likely be pursued by some desperate startups that really want to go beyond the homeland security market. Even then, however, patent issues can be formidable for such small companies because technical areas are well-covered by the major players in the industry.
China, however, is uniquely positioned to assume a major role in commercializing miniature MS analysis systems. Traditionally, instrumentation companies have not applied for patent protection for their technologies in China. Therefore, it is easier to produce a product package that includes the best suitable technologies in China than to do so in North America, Europe, Japan, or Australia. Would the size of the market in China justify such a development? Indeed it would. China has become the world's number-two market for MS products. Given the high cost of materials in the production-based economy, improper use of cheap materials and illicit additives is a problem in China that calls for product quality control. This is a major application area well suited for specialized MS systems. Since 2004, the Chinese government has invested in MS product development, and the funding amount was dramatically increased recently, with each individual project funded at $10M or higher. Although the direct product outcomes of these investments remain to be seen, the development activities have certainly trained many researchers and developers in the requirements of the MS instrument industry. Because it lacks a major player in that industry, China has been striving to establish one, and the concept of small instruments for specialized applications has long been considered as a good foothold for breaking into the business of MS manufacture. Also, the culture of "wide mass range," "high dynamic ranges," and "ultimately high resolution and precision" is not so deeply rooted in China as it is in other places. Thus it would come as no surprise to soon see some miniature MS products manufactured there and packaged into different systems suitable for various needs in different global regions.
References
(1) Z. Ouyang and R.G. Cooks, Annu. Rev. Anal. Chem. 2, 187–214 (2009).
(2) P.I. Hendricks, J.K. Dalgleish, J.T. Shelley, M.A. Kirleis, M.T. McNicholas, L. Li, T.-C. Chen, C.-H. Chen, J.S. Duncan, F. Boudreau, R.J. Noll, J.P. Denton, Z. Ouyang, and R.G. Cooks, submitted to Analytical Chemistry, 2013, under review.
(3) L. Li, T.-C. Chen, Y. Ren, P.I. Hendricks, R.G. Cooks, and Z. Ouyang, submitted to Analytical Chemistry, 2013, under review.
(4) L. Gao, A. Sugiarto, J.D. Harper, R.G. Cooks, and Z. Ouyang, Anal. Chem. 80, 7198–7205 (2008).
(5) J. Liu, R.G. Cooks, and Z. Ouyang, Anal. Chem. 85, 5632–5636 (2013).
(6) H. Wang, Y. Ren, M.N. McLuckey, N.E. Manicke, J. Park, L. Zheng, R. Shi, R.G. Cooks, and Z. Ouyang, Anal. Chem. 85, 11540–11544 (2013).
(7) H. Wang, J. Liu, R.G. Cooks, and Z. Ouyang, Angew. Chem., Int. Ed. 49, 877–880 (2010).
(8) Y. Su, H. Wang, J. Liu, P. Wei, R.G. Cooks, and Z. Ouyang, Analyst 138, 4443–4447 (2013).
(9) R.G. Cooks, Z. Ouyang, Z. Takats, and J.M. Wiseman, Science 311, 1566–1570 (2006).
(10) Z. Ouyang and X. Zhang, Analyst 135, 659–660 (2010).
(11) M.E. Monge, G.A. Harris, P. Dwivedi, and F.M. Fernández, Chem. Rev. 113, 2269–2308 (2013).
(12) Z. Takáts, J.M. Wiseman, B. Gologan, and R.G. Cooks, Science 306, 471–473 (2004).
(13) R.B. Cody, J.A. Laramee, and H.D. Durst, Anal. Chem. 77, 2297–2302 (2005).
(14) J.M. Nilles, T.R. Connell, and H.D. Durst, Anal. Chem. 81, 6744–6749 (2009).
(15) C. Berchtold, L. Meier, and R. Zenobi, Int. J. Mass Spectrom. 299, 145–150 (2011).
(16) I.X. Peng, R.R.O. Loo, E. Margalith, M.W. Little, and J.A. Loo, Analyst 135, 767–772 (2010).
(17) J.H. Kennedy and J.M. Wiseman, Rapid Commun. Mass Spectrom. 24, 309–314 (2010).
(18) L. Gao, R.G. Cooks, and Z. Ouyang, Anal. Chem. 80, 4026–4032 (2008).
(19) J. Liu, H. Wang, N.E. Manicke, J.-M. Lin, R.G. Cooks, and Z. Ouyang, Anal. Chem. 82, 2463–2471 (2010).
Zheng Ouyang is an Associate Professor in the Weldon School of Biomedical Engineering at Purdue University (West Lafayette, Indiana). He has a research interest in developing instrumentation and applications for mass spectrometry, with results published in more than 110 peer-reviewed publications. He has received a number of awards including the Wallace H. Coulter Foundation Early Career Translational Research Award in Biomedical Engineering, a China National Natural Science Foundation Award for Distinguished Overseas Young Scholars, a USA National Science Foundation Early Career Award, the American Society for Mass Spectrometry Research Award, and the International Mass Spectrometry Society Curt Brunnée Award for outstanding contributions to the development of instrumentation for mass spectrometry.

Zheng Ouyang
Kate Yu "MS — The Practical Art" Editor Kate Yu joined Waters in Milford, Massachusetts, in 1998. She has a wealth of experience in applying LC–MS technologies to various application fields such as metabolite identification, metabolomics, quantitative bioanalysis, natural products, and environmental applications. Direct correspondence about this column to lcgcedit@lcgcmag.com

Kate Yu

Evaluating Body Odor Sampling Phases Prior to Analysis
April 23rd 2025Researchers leveraged the advantages of thermodesorption, followed by comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC×GC/TOF-MS), to compare and assess a variety of sampling phases for body odor.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










