Reshaping The Landscape
An introduction from guest editors, Koen Sandra and Davy Guillarme, on this special supple-ment from LCGC Europe focusing on recent developments in biopharmaceutical analysis.
Biopharmaceuticals have substantially reshaped the pharmaceutical landscape. These products are being developed at an explosive rate and have attracted great interest from both smaller biotech firms and big pharmaceutical companies. This has become especially visible during these COVID‑tormented times where all eyes are on biopharmaceuticals. Their success is driven by their efficacy in disease areas with a high‑unmet medical need such as oncology, autoimmune, and infectious diseases. Antibody‑based therapeutics, that is, monoclonal antibodies (mAbs), antibody-drug conjugates (ADCs), bispecifics, fusion proteins, etc. reign supreme, but the biopharmaceutical market is further populated by other products from proteinaceous and, more recently, nucleic acid, that is, DNA, RNA, and cellular origin. All with one common denominator, an enormous therapeutic potential with a layer of immense structural complexity highly demanding towards analytics. Since the first LCGC supplement on Advances in Biopharmaceutical Analysis, we have witnessed an inspiring creativity amongst peers, and this has resulted in many innovative tools for the detailed study of these ever more complex molecules. Today, these fascinating study objects are subjected to two- (2D), three- (3D) and even four‑dimensional liquid chromatography (4D‑LC), and are introduced in the mass spectrometer (MS) under native conditions, coupling historically MS-incompatible chromatographic modes such as ion-exchange chromatography (IEC), size-exclusion chromatography (SEC) or hydrophobic interaction chromatography (HIC), and are even separated using hydrophilic interaction liquid chromatography (HILIC) or on pillar-based chromatographic columns. We further witnessed the introduction of new MS‑sensitive glycan labels, alternative proteases (for example, IdeS), implementation of inert columns and instruments, and a revival of capillary electrophoresis (CE), amongst many others. Several of these innovations have been published in LCGC Europe’s “Biopharmaceutical Perspectives” column, which was inaugurated in 2017. With so many opportunities on the shelf, it is highly rewarding as a scientist to be involved in biopharmaceutical analysis today.
For the current supplement, we have selected several authorities, both from academia and industry, giving their view on different aspects of biopharmaceutical analysis. The first contribution by Füssl et al. explores chip‑based electrophoresis hyphenated to mass spectrometry for the characterization of ADCs. The described setup preserves higher-order structures and non‑covalent interactions offering enormous potential for the analysis of labile proteins such as cysteine-conjugated ADCs. This work further builds on earlier work from this group, hyphenating charge-based chromatographic separations to native MS.
Protein biopharmaceuticals are recombinantly produced in mammalian, yeast or bacterial expression systems. Next to the therapeutic protein, these cells produce endogenous host cell proteins (HCP) that can contaminate the biopharmaceutical product despite major purification efforts undertaken. Since HCPs can affect product safety and efficacy, they need to be closely monitored. Esser-Skala et al. metaphorically describe the analytical challenges associated with HCP monitoring and review recent advances in LC–MS and HCP-enrichment strategies that allow to cross over the 1-ppm barrier in HCP detection, amongst others.
While the first two contributions have their primary focus on protein analysis, the subsequent manuscripts introduce us into the fascinating world of nucleic acids. Christina Vanhinsbergh sets the stage by highlighting the analytical challenges and different separation methods for therapeutic oligonucleotides such as antisense (ASOs) and splice‑switching oligonucleotides (SSOs), short interfering RNA (siRNA), aptamers, and messenger RNA (mRNA).
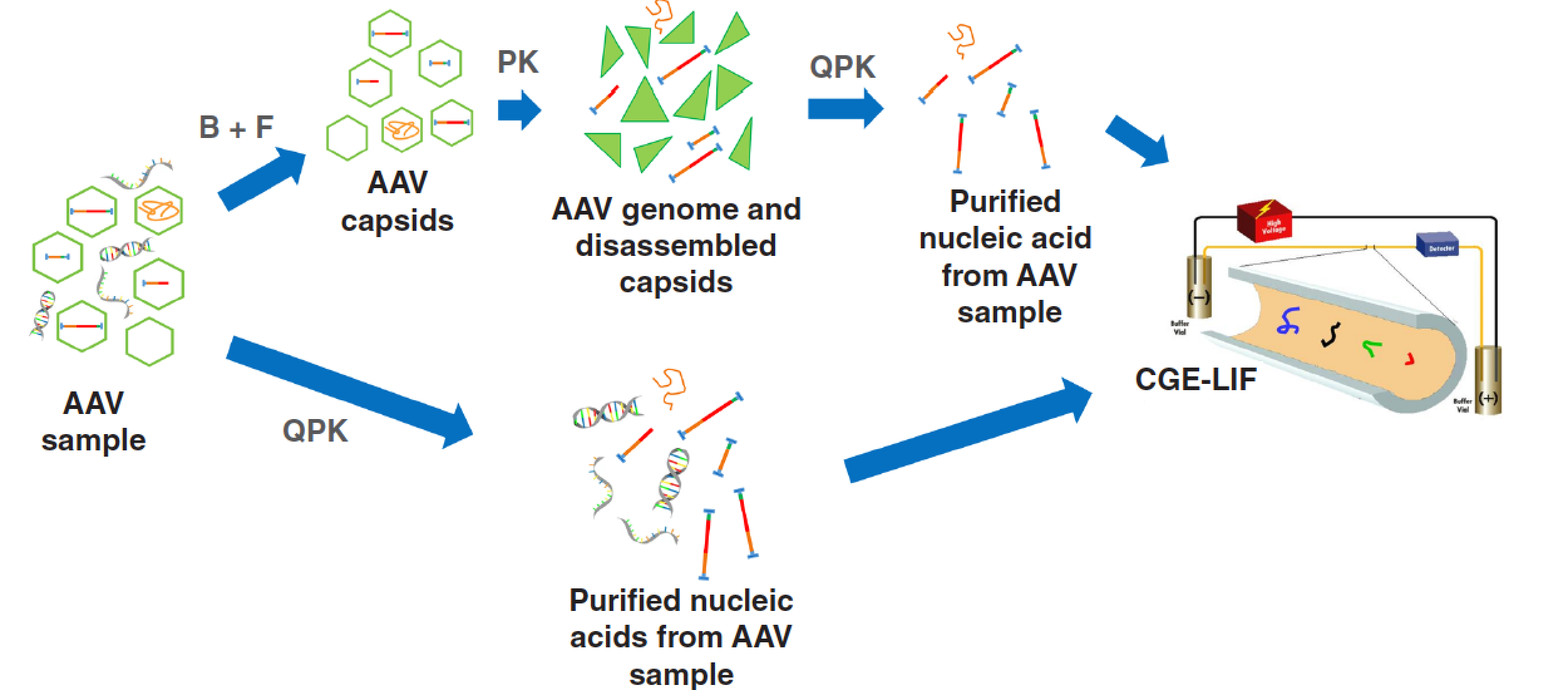
Zhang et al. discuss different physicochemical methods, including chromatography, CE, MS, and light scattering, but also imaging and spectroscopic techniques, for the characterization of viral vectors, including adeno-associated virus (AAV) and lentiviral vectors (LVV), and ancillary materials used in cell and gene therapy. The latter being a largely underexplored field for analytical scientists. Guttman and Luo further elaborate upon this topic, describing an elegant and rapid AAV genome integrity analysis method based on capillary gel electrophoresis (CGE).
It has been a great pleasure working together with renowned colleagues in the field and we would like to thank all of them for their excellent contributions. We are already eager to learn what advances future supplements will bring.

Koen Sandra is Director at RIC, Belgium and Visiting Professor at Ghent University, Belgium.
koen.sandra@richrom.com

Davy Guillarme is senior lecturer and research associate at the University of Geneva, Switzerland.
davy.guillarme@unige.ch

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.