Recent Improvements in Benchtop GC–MS
Special Issues
The 30-year history of advances in gas chromatography–mass spectrometry technology continues today. Recent improvements in hardware, electronics, and data analysis software have resulted in new levels of productivity and sensitivity that have broadened the potential applications for this laboratory mainstay.
The 30-year history of advances in gas chromatography–mass spectrometry technology continues today. Recent improvements in hardware, electronics, and data analysis software have resulted in new levels of productivity and sensitivity that have broadened the potential applications for this laboratory mainstay.
For three decades, gas chromatography–mass spectrometry (GC–MS) technology has undergone a continuous evolution of hardware, electronics, and software improvements. Capillary column interfaces, positive and negative chemical ionization (CI), use of turbomolecular pumps, automation of CI reagent gases control, 50- to 100-fold improvement in levels of detection, improved scanning speeds, enhanced automated data, and target compound analysis are just a few examples of improvements that have made the analysis of volatile and semivolatile compounds by GC–MS a routine and easy-to-implement technique. Given the maturity of the technology, many have assumed that the evolution of GC–MS is approaching its limit. However, important new developments in GC–MS suggest otherwise. This article will discuss how the new techniques of synchronous selected ion monitoring and scan acquisition, trace ion detection, deconvolution reporting software, and new ion source materials are significantly enhancing the productivity and sensitivity of benchtop GC–MS.
Combining Scan and SIM Acquisition Modes
The first GC–MS systems that were commercialized over 30 years ago had one mode of operation for obtaining mass spectral data — full mass spectrum acquisition. Mass spectrometers that used a quadrupole mass filter ramped the rf and dc voltages applied to the quadrupole in a continuum to acquire a mass spectrum. The fastest scan rates of systems produced in the 1970s were approximately 650 amu/s, and the primary goal of GC–MS systems at this early stage of their development was the generation of qualitative information for compound identification. For several years, GC–MS was not considered a quantitative analytical system because of its slow scan rates and lack of software to analyze quantitative information. That changed when two new developments were introduced: improved electronics and quantitative data analysis software. Instruments were developed with the electronic capability to adjust the rf and dc voltages to filter specific ions selectively in a predetermined cycle rather than scanning the entire mass range. The technique was called selected ion monitoring (SIM) and provided the capability to perform quantitative analysis using GC–MS. Because the mass spectrometer was being used to monitor a fixed number of specific ions rather than scanning an entire mass spectrum, the limit of detection for a compound was improved significantly because the duty cycle of the mass spectrometer was greatly improved. Picograms of compounds could be detected using SIM, in contrast to the nanograms required for a fully scanned spectrum. SIM also produced a very accurate quantitative profile of a compound being detected. Almost simultaneously, computerized algorithms were introduced that made the automated analysis of the quantitative data from SIM analysis feasible. These two developments started the development of quantitative analysis via GC–MS.
Generally, a GC–MS analysis was designed to be either qualitative (full scan) or quantitative (SIM only). GC–MS electronics did not have the bandwidth required to quickly change the rf and dc voltages needed to acquire both qualitative (scan) and quantitative (SIM) data synchronously during the same GC–MS analysis. However, GC–MS electronics have been improved significantly in the last few years. Current instruments can scan a spectrum at 10,000 amu/s (versus 650 amu/s in the 1970s) and switch between ions during an SIM analysis in 1 ms. These new capabilities allow synchronous collection of scan and SIM data (Figure 1) during an analysis without compromising the quality of data in either mode. No longer does a GC–MS method have to be developed to collect either qualitative or quantitative data, as both types of data can now be acquired during one analysis.

Figure 1
A method that is designed primarily for qualitative analysis using full-scan spectra can now be combined with SIM acquisition to provide quantitative information on selected compounds via SIM, with significantly improved sensitivity. In an analogous manner, an experiment that is designed primarily for quantitative information via SIM can have full-scan data incorporated into the method. One benefit of this SIM–scan combination is that qualitative information can be collected during a SIM–scan analysis that helps in identifying coeluted compounds that could interfere with the quantitative SIM analysis. The coelution of matrix compounds from a complex sample that could interfere with a SIM analysis could be detected easily and confirmed via the scanned spectrum.
One challenge of the SIM–scan acquisition mode that has been addressed is the generation of the optimal SIM m/z acquisition parameters. Software programs have been written that convert a full-scan acquisition file into the appropriate m/z and retention time groupings for a SIM analysis. Generating these parameters is done automatically and typically takes a few seconds to complete the conversion — a quantum leap compared to the manual methods of dividing up the chromatogram by "hand."

Figure 2
The benefits of a SIM–scan method compared with a scan-only method are demonstrated in the analysis of volatile organic compound (VOC) target gases, USEPA Method 8260. Figure 2 shows the first 2 min of a reconstructed total ion chromatogram (RTIC) for a VOC sample obtained using a SIM–scan acquisition mode. The scan and SIM RTICs of the SIM–scan method look very similar, indicating no loss in signal. However, a closer examination of peak 5, vinyl chloride, Table I, shows that the SIM data has a 10-fold improvement in the root mean square (rms) signal-to-noise ratio (S/N) when compared with the full-scan data from the SIM–scan acquisition or from a scan-only method.

Table I: SIMâscan and scan only sensitivity comparison. SIM data shows a 10-fold improvement in S/N
The faster data-acquisition electronics in modern GC–MS make fast chromatographic analysis feasible with a quadrupole-based GC–MS system. Capillary columns with internal diameters of 100 μm can produce peaks with one-half height widths of 180 ms and should contain more than eight data points for acceptable quantitation. Figure 3 shows the peak profile that was obtained using a 3-ms dwell time/ion. A total of 15 data points were obtained for this peak while three other SIM ions were being acquired during the analysis.
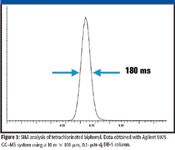
Figure 3
Trace Ion Detection
Another area of GC–MS technology that has been improved recently is ion-detection algorithms. The algorithms that analyze a mass spectrometer's raw data and generate mass and abundance pairs, perform the analysis in real time using algorithms that are coded in the mass spectrometer's firmware. Several groups have published algorithms to improve the S/N and chromatographic resolution in complex chromatograms, but most were run after a sample had been collected (1).

Figure 4
Advances in microprocessor technology and software algorithms have now made the real-time analysis of complex matrices possible. One example is the Trace Ion Detection algorithm (Agilent Technologies, Palo Alto, California), which improves the analytical quality of the data by removing spectral noise and chemical artifacts in the mass spectral data. One of the primary benefits of this approach is improved analysis of compounds in complex matrices. The performance of the algorithm is illustrated in Figures 4 and 5.
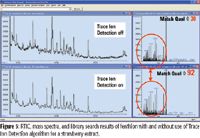
Figure 5
Deconvolution Reporting Software
One of the major applications of GC–MS is target compound analysis. The detection and quantitation of known compounds in complex samples is a common application for many segments of GC–MS users. The analysis of foods, air, water, and drugs of abuse for target compounds is a routine analysis done thousands of time every day. Analytical laboratories, like other segments of industry, are constantly being challenged to improve their efficiency and reduce costs. One way to address this challenge is to automate the analysis of complex chromatograms through the use of software that performs unattended analysis in minutes compared with hours by human efforts. This can be achieved by combining the Automated Mass Spectral Deconvolution and Identification System (AMDIS) with databases that contain target-compound retention times. AMDIS was developed by NIST (Gaithersburg, Maryland) for the detection of chemicals that are in violation of the Chemical Weapons Convention (2). One of its primary goals is to identify target compounds in complex matrices. AMDIS deconvolves complex chromatograms with overlapping peaks into their individual spectra and performs a library search using a database of 147,000 compounds from the NIST library or a user-generated or commercially supplied database. The utility of AMDIS can be enhanced significantly by combining it with databases that include compound retention times. The AMDIS software does not perform any quantitative analysis of the data. However, by incorporating a database that contains qualifying ion ratios for three ions of a target compound and an internal standard calibration table, an estimate of compound amounts can be obtained. Combining the two reports produces a qualitative and quantitative analysis for the target compounds.
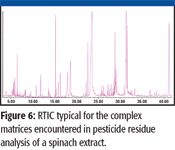
Figure 6
The analysis of a spinach extract is shown in Figure 6. Typically, a human operation would take 45–60 min to analyze the extract and perform a library search for the major components. Using the combined AMDIS and a retention time and spectral library of 567 pesticides, the report shown in Figure 7 was produced in 1 min. Additional compound libraries are available for volatiles, semivolatiles, PCBs, and pesticides.
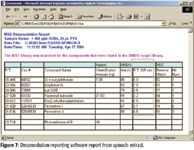
Figure 7
Ion-Source Materials
Various stainless alloys have been the material of choice for ion-source manufacturing for many years. Although stainless steel alloys have many favorable properties for ion sources, such as resistance to oxidation and corrosion and being nonmagnetic, they also can react with many organic compounds at high temperatures and produce deleterious effects. This could be demonstrated by comparing an electron-capture detector chromatogram with an RTIC produced by an electron ionization source. The RTIC often would produce a very asymmetrical peak with excessive tailing, and the mass spectrum of the compound would contain peaks that were indicative of thermal reaction between the sample and stainless steel. Figure 8 illustrates the abundant 247 ion that originates from a reaction between the compound and the source. Use of new ion-source materials that are constructed from inorganic conductive materials containing nitrides and disulfides of metals has reduced the decomposition of reactive compounds significantly in the ion source. Figure 9 shows the virtual elimination of the m/z 247 ion that was indicative of source interactions. An additional benefit of the new ion-source material is an increase in sensitivity for reactive compounds. Compounds containing phosphorus and sulfur moieties have shown 50–100% increases in response using the inert source material.
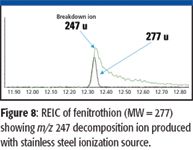
Figure 8
Conclusion
Significant improvements in the hardware, software, and electronics used in modern GC–MS systems have led to improved scan and SIM acquisition parameters, quantitation, spectral fidelity, and sensitivity and have allowed higher productivity. As GC–MS systems have become easier to operate and capable of analyzing complex data sets faster, they are becoming an alternative to the use of selective GC detectors that have been used for many years to achieve selectivity and sensitivity.
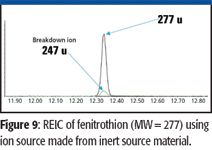
Figure 9
Monty Benefiel and Harry Prest are with Agilent Technologies in Santa Clara, California.
References
(1) A. Ghosh and R. Anderegg, Anal. Chem. 61, 2118–2121 (1989).
(2) S.E. Stein, J. Am. Soc. Mass Spectrom. 10, 770–781 (1999).

Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.












