The Reality of Lab-on-a-Chip Technology for the Mass Spectrometry Laboratory
LCGC North America
In this installment of "MS-The Practical Art," guest columnist Jack Henion presents an historical and practical overview of lab-on-a-chip (LOC) research and the commercial achievement associated with it so far.
I first discovered the promised potential of lab-on-a-chip (LOC) technology at a Gordon Conference in the mid-1970s, when gas chromatography (GC) columns were reported on glass microscope slides. Since then, the technology's potential has fueled much innovation and product development. Yet this potential has not for the most part transmuted into practical, commercially available products. Although the widespread appeal of LOC generates much publicity and excitement, manufacturing a robust, reliable, and user-friendly device that is as good as or, ideally, better than established technologies has proven elusive. The technology's challenge, it seems, is twofold. One factor is technical: the difficulty (so far) to efficiently transfer "real-world" samples to the chip or miniature device. The other factor is human: our inborn resistance to accepting change, particularly when the perceived advantages of doing so are but minimal.
Certain separation techniques, including capillary electrophoresis (CE) and nano high performance liquid chromatography (HPLC), have been performed on a chip. These techniques use both on-chip and off-chip detection. However, when a mass spectrometer serves as the detector, additional practical challenges arise. It is relatively easy to focus a laser through a glass-microfabricated channel for on-chip, laser-induced, fluorescence detection (1). But it is quite another matter to remove the sample from the chip and thereafter ionize and transport the sample for mass spectrometry (MS) detection. Nevertheless, despite this difficulty, reports continue to suggest a future for chip-based analytical devices (2–4). The actual measure of the technology's success, however, will take the form of commercial LOC products that customers choose and use because they work better than established techniques.
Why microchips appear to make sense: To those who grapple daily with the challenges of conventional laboratory methods, the concept of integrated chemical and analytical processes that share a common substrate free of sample transfers and associated plumbing has much to recommend it. You can discard an LOC device after a single or otherwise limited use. Doing so obviates the need to wash glassware and frees you from concern about sample-to-sample carryover. It also makes possible low-cost, easy-to-follow procedures. Moreover, from the LOC–MS point of view (5), the volume of liquid handled is nicely compatible with mass spectrometer operation. For both electrospray ionization (ESI) and matrix-assisted laser desorption ionization (MALDI)-MS, the lower the flow, the better the analysis. Thus, MS, an easily scalable technique, exhibits an enhanced response when you decrease the sample size (5). Unfortunately, some chip-based commercial products currently fail to impart such advantages.
Why microfabricated devices are difficult to use: Given the relatively large surface-to-volume ratio of microfabricated channels compared to that of conventional HPLC columns, analytes are far more likely to interact with the capillary wall. Indeed, in the trace quantities typically used, they can adsorb to the wall's surface or become otherwise lost, to unexpected chemistry or accelerated degradation. Another barrier to the straightforward use of microdevices lies in their limited sample capacity. An analysis of, say, a biological sample can fail because the trace analytes of interest are in the presence of high levels of matrix components. Thus, loading the "dirty" extract into a microchannel can overwhelm the intended processes — chromatographic selectivity, solid-phase extraction (SPE) cleanup, and so forth — such that the intended processes might not occur. Finally, that same limited sample capacity necessitates sensitive detection capabilities which, in some cases, challenge the detection limits of modern mass spectrometers.
The dream for LOC–MS is a fully integrated device that integrates sample handling, sample preparation, and separation science (CE, HPLC, and so forth) coupled to a mass spectrometer as a sensitive, selective detector. Additional promises include the ability to handle toxic chemicals safely, economically, and in an environmentally responsible way. Low sample and reagent quantities and improved detection limits for concentration-sensitive detectors make these promises worth pursuing, as does the reduced requirement for operator intervention and the need to connect tubes, fittings, or accessories.
In principle, the LOC device should be inexpensive, portable, disposable, and amenable to productive use by unskilled operators. Regrettably, LOC analytical devices do not couple well with much larger instruments such as mass spectrometers. So the promise implied by the devices is difficult to deliver on. Yet progress is ongoing, and hope remains for the future of LOC–MS and related technologies.
Progress to Date: Coupling the "Real World" to Microtechnology
Sample handling and preparation: Before most samples can be analyzed, they usually must be "cleaned up" via a process that often includes extracting and concentrating the targeted sample analytes. LOC technology is no different. You must load a crude sample (biological, environmental, industrial, and so forth) onto the LOC device for extraction and sample cleanup. This "real-world-to chip" interface is a simple concept, but it presents a considerable challenge. A few innovative approaches (see the following text) have been commercialized, but on this aspect of LOC development, more progress is needed.
When you load a sample onto a chip, and then prepare it by SPE or other form of extraction, you must afterward transfer the extract to the next stage of the analysis process. Working in our laboratory at Cornell University (Ithaca, New York), my colleagues and I reported an early attempt to do so. We constructed a parallel array of eight microporous, monolithic, SPE sorbents in a polymer (Zeonor) chip to accommodate a serial, microfluidic, SPE sample cleanup of eight separate, biological samples before being directly coupled with electrospray MS (6) (Figure 1).
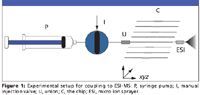
Figure 1
From 10-μL aliquots of human urine fortified with imipramine and imipramine-d3 (internal standard), we obtained acceptable recoveries, quantitative precision, and accuracy from ESI-MS measurements at levels ranging from 0.025 to 10 μg/mL. We cleaned up the biological samples, passing them through the integrated, microfabricated, porous, SPE monolith using a syringe pump followed by analyte elution of the SPE extract directly to the ESI-MS system. This approach precludes an HPLC step, which is, in some instances, possible when the SPE cleanup step is adequately selective for the targeted analytes. One such instance would be when the MS step affords the tremendous selectivity of a tandem mass spectrometer operated in the selected-reaction-monitoring (SRM) mode and targeting unique fragmentation transitions for the analytes of interest.
In a related experiment, we reported on-chip P450 metabolism in a microfabricated, serpentine channel where reagent solutions were delivered and mixed on the chip via an external syringe pump (Figure 2). Thus, imipramine was converted to desipramine via a chip-based P450 biotransformation, and the IC 50 value for the chemical inhibitor was determined by ESI-MS (7). We cleaned up the enzymatic product mixture using a chip-based, integrated, porous, monolithic SPE cartridge followed by direct analysis by ESI-MS. Figure 2 shows a schematic diagram of the syringe inlet ports directed to the enzymatic reaction region, which couples to the monolithic SPE cartridge in the chip, where the product mixture is desalted and cleaned up. After eluting the unwanted substances from the SPE cartridge, syringe pump C (Figure 2) delivers the elution solvent–spray mixture for direct ESI-MS analysis.
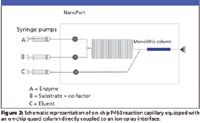
Figure 2
Despite the apparent success of the reported results, relatively few additional reports on this concept have come to light. Hence, we have seen little commercial development of this approach even though in a "perfect world" it would seem logical to develop such an integrated micro-sample cleanup analysis system.
A truly successful micro-sample preparation system that is also user friendly could preclude sample losses, analyte degradation, and excessive waste of solvents. It also could reduce the use of expensive reagents and enzymes and the time required to prepare samples for analysis. As to why a commercially viable, integrated approach has not emerged, the most likely reasons are a lack of confidence on the part of manufacturers in a strong commercial market and a lack of convincing evidence that LOC concepts are faster, better, and cheaper than conventional procedures.
Chip-based analytical separations: Most of the reports for chip-based separation science involve just one or two operations actually performed on the chip. These operations can include CE or HPLC separation of a chemical mixture, most often with spectroscopic detection performed on the chip. In practice, it is far easier to effect a CE separation-detection operation on a chip because the only external connections required are electrodes, which attach to the chip's microfluidic reservoirs. Under an applied high voltage, the electrophoretic separation occurs with or without electroosmotic flow, which depends upon the pH of the medium and the surface chemistry of the microchannel. The small volumes of fluids needed are well suited for the CE technique and detection by electrospray MS.
An early report established that one can couple rapid, chip-based, CE separations of acylcarnitines with electrospray MS (8). Nevertheless, little significant progress has been made toward developing a commercial CE–MS product that adopts LOC technology (Figure 3).
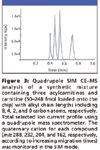
Figure 3
In contrast to the relative ease of CE separation, chip-based HPLC separation is far more challenging because of the technology involved in microfluidic pumping of HPLC mobile phases. Tempting though it might be to simply connect an external nano-HPLC pump via capillary tube connections and fittings, doing so would mean losing the portability and simplicity of a true chip-based system. At the recent ASMS Conference on Mass Spectrometry, a poster presentation by Eksigent Technologies, Inc. (Livermore, California) (9) described such an approach with its recently introduced commercial cHPipLC Nanoflex system. This glass chip contains etched, microfabricated channels packed with C18 reversed-phase packing, which accommodates a direct coupling from a nano-HPLC pump, delivering mobile phase to the chip. This commercial system reportedly makes nano-LC–MS easier and more reproducible. For nano-LC–MS applications, the exit of the glass, chip-based packed capillary column connects via a fused-silica capillary to a nonintegrated nanoelectrospray emitter, which is directed to an atmospheric pressure ionization (API) mass spectrometer. High-quality LC–MS data are then presented for an enzymatic digest (Figure 4). Yet it remains to be seen whether routine LC–MS users will embrace this commercial idea for either small- or large-molecule applications.

Figure 4
When she was with Barry Karger's research group at Northeastern University (Boston, Massachusetts), Prof. Iulila Lazar successfully confronted the challenge of on-chip pumping (10). Two sets of multiparallel nanochannels produced a hydrodynamic-gradient, HPLC flow directly on the chip, effecting the separation and analysis of enzymatic digests. Such work represents the kind of innovation needed to produce independent, chip-based, HPLC technology. However, the technology must pass the practicality test of commercial manufacturing and end-user success for routine problem solving. Prof. Lazar's research continues at the Virginia Polytechnic Institute (Blacksburg, Virginia) in the Virginia Bioinformatics Institute (11). To date, though, there appears to be little effort to commercialize this approach to LC–MS-on-a-chip for ESI-MS.
More recent commercial developments in the area of chip-based LC–MS originated from Agilent Technologies (Santa Clara, California) several years ago. The Agilent HPLC Chip Cube is a novel, integrated, two-dimensional HPLC column system formed from a disposable chip of polyimide substrate that includes an integrated electrospray emitter at the terminus of the HPLC packing material. This nicely integrated chip-based device is easily introduced manually into the center of a special HPLC injector. The injector accommodates sample loading and injecting as well as gradient formation of mobile phase fed from a modern external nano-HPLC pumping system and its associated autosampler. This arrangement precludes any plumbing connections by the user and nicely provides an interface for transferring sample between the real world and the chip world via the autosampler. The Chip Cube, commercially available for the past several years, is currently usable only with the Agilent electrospray mass spectrometer product line. Convincing reports of this system's performance have appeared in the recent literature (12). Figure 5 denotes those portions of the Agilent chip that correspond to conventional non-chip components of a conventional nano-LC–MS experiment.
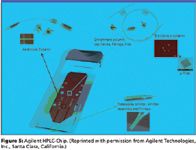
Figure 5
More recently, Waters Corporation (Milford, Massachusetts) introduced its Trizaic ceramic, or "nanoTile", chip. Although the chip parallels Agilent's Chip Cube concept, it nevertheless does so with some interesting differences. The company's UPLC technology device uses a ceramic microfluidic device packed with Waters' sub-2-μm chemistries to provide high-peak-capacity separations and increased usability. The nanoTile substrate is available in scalable formats whose internal diameters range from 75 μm to 300 μm and make them ideal for use in proteomics, biopharma, and small molecule pharma separations. Figure 6 shows a comparison between a modern nano-UHPLC column and the nanoTile chip for the LC–MS analysis of a tryptic digest of E. coli. This device can withstand sustained use. Moreover, it is easy to use, this according to its developers who claim they eliminated the steep learning curve that affects new users vis-à-vis adjusting fittings and affixing emitters on the nano-scale. As of this writing, Waters has not yet launched the technology. In any event, whether it receives widespread acceptance and commercial success remains to be seen.

Figure 6
Prof. M. Ramsey and colleagues (University of North Carolina, Chapel Hill) (13) reports an elegant, chip-based, research approach that describes a two-dimensional nano-HPLC–CE combination on a single chip. It incorporates capillary LC coupled to an integrated lossless sample transfer for electrophoretic separation and subsequent ESI via nanoelectrospray. The report describes nano-LC coupled serially to CE with ESI-MS detection(13). The experiments are conducted by rapid, repetitive, CE sampling from the relatively longer nano-LC chromatogram into a much shorter CE channel (for rapid separation). The process effects an electrophoretic separation within seconds, followed by the next CE sampling, taken from the nano-LC chromatogram as the latter progresses. The effluent from the CE capillary is directed continuously to the nanoelectrospray emitter, which is microfabricated from the corner of the glass chip to provide real-time ESI-MS data from this multidimensional system. Given the tremendous analytical horsepower of truly orthogonal separation mechanisms, such as HPLC and CE coupled with ESI-MS detection, the chip-based integration of these techniques would command high merit were they ever commercialized and made easy-to-use.
Such a commercially successful product would necessarily be fully automated and easy to use before scientists working on real-world problems would embrace it. Other issues remain, such as those involving proper loading of complex or dirty samples onto the nano-LC column and acceptable CE running buffers that are "MS-friendly," but for each a solution exists, making this approach potentially viable and competitive in the future.
Miniaturization of MS: Although chip-based mass spectrometers have been reported, this paper will not focus on this topic because commercial products based upon analytical applications employing condensed phase separation sciences appear to be quite a ways off in the future. The current systems employ electron ionization (EI) and sample inlets via GC. Readers interested in chip-based mass spectrometers should refer to recent reports (14–16).
Chip-based nanoelectrospray: Advion BioSciences (Ithaca, New York) addresses the real-world-to-chip issue in a simple and novel way (Figure 7). The company developed what it calls the ESI-Chip from a silicon substrate and commercialized it paired with a specially designed robot that delivers samples via a common disposable pipette tip from a microtiter plate to the chip (17). The robot can access as many as 400 samples serially during unattended ESI-MS analysis.

Figure 7
Because most modern laboratories routinely use either 96-well or 384-well plates as sample holding devices for LC–MS and related applications, Advion developed the robotic TriVersa NanoMate system to accommodate the multiwell plate format. Instead of using an HPLC autosampler to access samples in a multiwell plate, the NanoMate system is programmed to access each of the wells in succession using a disposable pipette tip for each sample. The sample selection step involves aspirating an aliquot of sample solution from a targeted well in the plate and then delivering the solution to the back of an emitter microfabricated in the ESI-Chip, where it undergoes automated nanoelectrospray-MS analysis. The chip consists of a 20 × 20 array of microfabricated nano-ESI emitters (18) placed in sequence close to the ion-sampling orifice of an API mass spectrometer. The emitter and the pipette tip are single-use items that preclude the possibility of sample-to-sample carryover. If the samples within the multiwell plate are sufficiently cleaned up by a selective sample extraction technique, this infusion-MS process can provide a rapid (30–45 s/sample) analysis of each sample in the plate, without chromatography. The strengths of this system include automated nanoelectrospray, zero carryover between samples, and increased sensitivity with reduced matrix suppression, afforded by nanoelectrospray (19). In addition to the original simple infusion experiments, the system also can perform an LC–MS analysis, using a special LC coupler to the microfabricated chip emitter, while simultaneously collecting fractions into a multiwell plate using the TriVersa NanoMate system. Following an LC–MS-MS analytical run, you can program the system to return to targeted collected fractions (wells) for additional infusion MS-MS experiments, as frequently done in drug metabolite identification or peptide–protein identifications.
Initial commercial acceptance of this technology was slow, but several novel applications have evolved. These include metabolite identification, protein sequence coverage (measuring biospecific noncovalent interactions), monitoring for chemical terrorism agents, profiling crude oil chemical composition (petroleomics), and characterizing and quantifying lipids (lipidomics). Although one could imagine potential for this technology in quantitative bioanalytical MS-MS applications, the relatively high cost of the additional hardware, as well as the consumable cost of the single-use chip, have contributed to the technology's lack of "market pull."
Modern bioanalytical SRM LC–MS techniques are difficult to displace because the established technology works so well. In addition, most practitioners in small molecule measurements have been slow to adopt nano-ESI and other "nano" techniques, so a reluctance to adopt new technologies prevails absent truly compelling benefits. In contrast, the future potential for LC–MS applications in clinical diagnostics could prove significant. Rapid, infusion-MS techniques may well satisfy the need to analyze very high sample numbers (millions per year) for certain tests, which are now performed using LC–MS techniques. Supporting that prediction is a report on chip-based, infusion-MS determination of carnitines prefractionated off-line as dried blood spots for newborn screening using the TriVersa NanoMate system described previously (20).
Innovative, simple, and practical LOC technology: A recent creative and simple approach to miniaturizing analytical chemistry for diagnostic purposes tends not toward LOC but "lab on a piece of paper and a cell phone." Prof. George Whitesides and his colleagues at Harvard University (Cambridge, Massachusetts) recently reported a variety of very simple devices using common materials rather than sophisticated chips microfabricated from silicon, polymer, or glass substrates (21). The do-it-yourself diagnostic kit does not yet include integration with MS, but it can be used in developing countries or in remote locations where placing specially trained personnel is difficult and costly. The Harvard investigators have described patterning Whatman paper with a photoresist to create a microfludic device with a central channel and four side channels leading to wells for different colorimetric assays (Figure 8). Whatman paper wicks fluids well, so it can soak them up without an external pumping source, and it filters out particulate material as well. Such features readily and simply solve some of the problems associated with on-chip movement of fluids and minimizing particulate blockages in microchannels. Reagents for glucose and protein assays are embedded within the wells. You can run both tests using visible oxidation reactions (wherein a camera-phone photographs the test results) and then send the images to a central facility where experts interpret the data and recommend treatment. Although not yet thoroughly field-tested, this is a very creative and reasonable approach to implementing miniaturized chemistry or biochemistry with simple materials. Regarding integrating mass spectrometry with this technology, you could possibly use direct analysis in real time (DART) (22), desorption electrospray ionization (DESI) (23) or analysis of samples at atmospheric pressure (ASAP) (24), if a low cost mass spectrometer were to become available.

Figure 8
Dried blood spots as miniature devices: The previously described approach does not (for obvious reasons) employ a mass spectrometer. A developing, parallel strategy in the form of small, dried blood spots on Guthrie cards or high-quality filter paper, however, does. Dried blood spots have been used to screen newborns for inborn errors of metabolism (25) for more than a decade. Now this concept has rekindled interest within the pharmaceutical industry for pharmacokinetic measurements in drug development (26). Among the attributes of dried blood spots, which involve a form of miniaturization, are simplified sample collection, transport, and analysis. Following, usually, a one-step solvent extraction of the dried blood spot and, sometimes, a derivatization step, flow injection analysis–MS (FIA–MS) is performed to monitor a panel of targeted analytes. A recent novel report (27) showed direct extraction of analytes from small, dried blood spots using the TriVersa NanoMate system (vide supra) and surface sampling followed by chip-based nanoelectrospray MS. Other, related developments in this simple approach to sample collection and analysis, are possible in the near future, and those could be amenable to LOC techniques.
Although the latter two innovations do not directly involve chip-based technologies, it is easy to imagine an evolutionary process leading to such a capability. Key elements that they share are simplicity, low cost, and ease-of-use — all of which will in coming years drive wider acceptance of LOC and miniaturization (28).
Summary and Conclusions
A KISS — keep it simple, stupid! — product strategy, combined with a user-friendly interface and effective results may be what developers must first and foremost consider when pursuing new LOC concepts. To date, we have seen numerous reports of clever, microfabricated devices, and applications that purport to provide the promise of LOC benefits. Yet for the most part, we have seen only limited commercial success on the part of any company that has been bold enough to venture into the market offering LOC products. Although exciting to contemplate, "chips" have not been widely accepted yet in the commercial arena. Customers become enthusiastic about them at meetings and from novel literature reports of LOC developments. Yet LOC techniques have not demonstrated the compelling, cost-effective benefits required to displace current technology or workflow. Nor has it shown the ease of use needed to induce users to change. The major benefit claims of minimum sample requirement and solvent and reagent savings from LOC approaches seem encouraging. Ultimately, however, they are not so high a priority for most practitioners, who are not so constrained by considerations of sample waste that the benefit of acquiring LOC technology would clearly outweigh the burden of doing so. As for other waste, in most cases, scaling-down approaches such as micro- and nano-LC easily reduce solvent and reagent consumption to acceptable levels. Perhaps the mission to "go green" will help support LOC efforts, but for the foreseeable future, environmental considerations do not constitute a compelling reason for most to switch to chip-based approaches.
Good reasons nonetheless abound for embracing LOC developments for future implementation, commercialization, and success. Despite the merits of our current sample analysis capabilities, many deficiencies in our analytical strategies and workflow continue to plague us: sample transfer losses, extra-column variance in chromatographic separations, expenses related to excess solvent and reagent disposal, and detection limitations associate with sample dilution — to name but a few. Although our modern sample preparation-analysis procedures coupled with MS work fairly well, there is always room for improvement. Finally, for many laboratories, acquiring personnel whose skill levels are equal to performing experiments using complex equipment is challenging. LOC techniques could lessen the challenge by integrating some procedure steps on an automated chip.
Given that human nature disposes us toward risk averseness or laziness, we often find insufficient reason to change our ways without compelling grounds to do so. Thus, commercial success of LOC products that integrate MS will await a day when such products prove themselves easy to use, robust, reliable, and economically viable.
Acknowledgement:
The author wishes to thank Dr. Kathryn H. Henion for her valuable assistance in editing this manuscript and reproducing some of the figures.
Dr. Jack Henion is Emeritus Professor of Toxicology at Cornell University, Ithaca New York, where he was a member of the College of Veterinary Medicine from 1976 to 2006. He is also a co-founder of Advion BioSciences, where he serves as Chairman and Chief Scientific Officer. From 1993 to 2006, he served as Chairman, President, and CEO.
Michael P. Balogh "MS — The Practical Art" Editor Michael P. Balogh is principal scientist, MS technology development, at Waters Corp. (Milford, Massachusetts); a former adjunct professorand visiting scientist at Roger Williams University (Bristol, Rhode Island); cofounder and current president of the Society for Small Molecule Science (CoSMoS) and a member of LCGC's editorial advisory board.

Michael P. Balogh
References
(1) L.M. Ramsay, Anal. Chem. 81(5), p. 1741–1746 (2009).
(2) C.A. Henry, C&E News. 14–19 (2007).
(3) R.T. Kelly and A.T. Woolley, Anal . Chem, 96A-102A (2005).
(4) C.M. Harris, Anal. Chem., 64A-69A (2003).
(5) S.L. Gac and A.v.d. Berg, eds. Miniaturization and Mass Spectrometry. 2009 , RSC Publishing: Cambridge CB4 0WF, UK. 315.
(6) A. Tan, S. Benetton, and J. Henion, Anal. Chem. 75, 5504–5511 (2003).
(7) S. Benetton, et al., Anal. Chem. 75, 6430–6436 (2003).
(8) Y. Deng, H. Zhang, and J. Henion, Anal. Chem. 73, 1432-1439 (2001).
(9) J.B. Young, et al. High capacity separations for complex proteomic mixtures using multiple microfluidic chip columns in series. in American Society for Mass Spectrometry (ASMS). 2009. Philadelphia, PA.
(10) I.M. Lazar and B.L. Karger, Anal. Chem. 74(24), 6259-6268 (2002).
(11) J.M. Armenta, A.A. Dawoud, and I.M. Lazar, Electrophoresis 30, 1145–1156 (2009).
(12) M.H. Fortier, M.-H., Anal. Chem. 77(6), 1631–1640 (2005).
(13) J.S. Mellors, et al., Anal. Chem. 80(18), 6881–6887 (2008).
(14) D.E. Austin, et al., Anal. Chem. 79(7), 2927–2932 (2007).
(15) S. Wright, et al., JASMS 20(1), 146–156 (2009).
(16) L. Gao, et al., Anal. Chem. 80(19), 7198–7205 (2008).
(17) G. Schultz, T. Corso, and S. Prosser, "Monolithic nanofabricated nanoelectrospray device from a silicon substrate," in Proceedings of the 47th ASMS Conference on Mass Spectrometry and Allied Topics, ASMS, Editor. 1999, American Society for Mass Spectrometry: Orlando, FL.
(18) G. Schultz, T. Corso, and S. Prosser, Anal. Chem. 72, 4058–4063 (2000).
(19) E.T. Gangl, et al., Anal. Chem. 73(23), 5635–5644 (2001).
(20) D.H. Chace, J. Mass Spectrom. 44, 163–170 (2008).
(21) A.W. Martinez, et al., Anal. Chem. 80(10), 3699–3707 (2008).
(22) C. Petucci, et al., Anal. Chem. 79(13), 5064–5070 (2007).
(23) I. Cotte-Rodriguez, et al., Anal. Chem. 77(21), 6755-6764 (2005).
(24) C.N. McEwen, R.G. McKay, and B.S. Larsen, Anal. Chem. 77(23), 7826–7831 (2005).
(25) D.H. Chace, T.A. Kalas, and E.W. Naylor, Clin. Chem. 49(11), 1797–1817 (2003).
(26) N. Spooner, R. Lad, Anal. Chem. 81(4), 1557–1563 (2009).
(27) V. Kertesz and G.J. van Berkel, "Ambient Surface Sampling Mass Spectrometry Using a Fully Automated Chip-Based Nano-Electrospray System," 2009. Philadelphia, PA: ASMS.
(28)H. Becker, Lab Chip 9(15), 2119–2122 (2009).

Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)




