Questions from Afar
Retention-time drift, temperature control and broad peaks from a new column are the topics of this month's discussion.
As many of you know, a major part of my job is teaching liquid chromatography (LC) training courses around the world. Besides getting to see some pretty fascinating places, I get to meet lots of people and have a chance to help solve some LC problems that are troubling the attendees. One thing I have found is that chromatographers everywhere tend to have the same struggles with LC problems. Sometimes I feel a bit like the detective on Garrison Keillor's A Prairie Home Companion, whose radio dramas always end with the tag line, ". . . one man is trying to find answers to life's persistent questions . . . Guy Noir, Private Eye." In this month's instalment I'd like to share a few of the questions that came up in recent classes.
Retention-Time Drift
One attendee mentioned a problem related to a small but noticeable increase in retention time that continued to grow over several weeks. In an earlier instalment (1) we looked at tricks to help determine the cause of retention drift. One of the ways to differentiate between hardware (flow-rate) problems and chemical (column, mobile phase or temperature) problems was to see if the disturbance at the column dead time changed in the same proportion as the retention change. If it did, a hardware problem was indicated; otherwise it was a chemical problem. However, the current problem was observed on an LC system with a mass spectrometry (MS) detector. In contrast to ultraviolet (UV) detectors, where a solvent-front disturbance is almost always seen, unless the MS system is set to specifically look for ions at the dead time, the baseline is flat in this region. As a result, the dead time diagnostic didn't help.
The first step taken was the easy one — replace the column. When this approach didn't help, a new batch of mobile phase was made, but it didn't solve the problem either. Next came cleaning the pump check valves and finally replacement of the pump seals. None of these changes arrested the drift.
Then one day, the user was inspecting the autosampler and noticed a reflection from a drop of liquid on the injection valve. This led him to service the valve. He discovered that there was a scratch between two of the passages in the valve rotor, creating a situation called cross-port leakage. A tiny amount of mobile phase was leaking out the waste line from the injector, effectively lowering the flow-rate to the column and thus increasing the retention times. As the leak became worse over time, the loss of flow increased and retention times drifted to even longer times. Replacement of the rotor seal corrected the problem. Because the leak was small, likely only in the tens of microlitres per minute, and a piece of opaque tubing was used to route the injector waste to the waste container, the leaking solvent was not noticed. Replacement of the valve rotor corrected the problem.
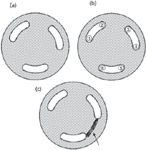
Figure 1: (a) Injection valve rotor showing polymeric seal (shaded) and grooves to pass liquid (clear). (b) Typical plumbing connections: 1 and 4, injection loop connections; 2, to column; 3, to pump; 5, to waste; 6, sample inlet. (c) Lines showing scratch between ports 4 and 5 (arrow). Adapted from Figure 17.3 of reference 2.
The problem can be more clearly understood with the help of the valve rotor sketches in Figure 1. The rotor usually comprises a polymer disk typically 15–30 mm across and 5 mm thick, with three semicircular grooves cut into the surface as shown in Figure 1(a). In operation, a mating stationary piece (the stator) contains connections to the sample loop (ports 1 and 4), the column (port 2), pump (port 3), waste (port 5), and sample inlet (port 6) [Figure 1(b)]. The configuration of Figure 1(b) is in the inject position, where the loop is in the flow path. In the load configuration (not shown), the rotor would be rotated 60° to the right and the loop would be connected to the sample inlet and waste, while the pump would feed directly to the column. The valve rotor has a very long lifetime under normal conditions — I've had numbers of 100,000 to 500,000 cycles quoted to me by instrument manufacturers — so routine replacement of the injector rotor is not something that most of us ever encounter.
However, if a tiny piece of hard material gets caught in the injector, it can scratch the rotor, as illustrated in Figure 1(c). Now some of the mobile phase can leak from the injection loop to waste instead of 100% of the flow going to the column. When such problems occur, they are most likely caused by a hard, insoluble particle in the sample. Centrifugation or filtration of the samples before injection should avoid this problem. I have also seen a case where a hand-cut piece of tubing was not rinsed properly, resulting in a tiny sliver of stainless steel working its way into the valve and scratching the rotor. Whenever tubing is hand-cut, whether it is stainless steel or plastic tubing, be sure to rinse it before use. The easiest way to do this is to connect the upstream end of the tubing and turn the pump on for a minute or two to flush the tubing to waste before connecting the downstream end.
Temperature Control
One of the topics that comes up regularly in this column is the importance of controlling the column temperature. Retention times can change by 1–2% for each 1 °C change in the column temperature, and peak spacing can change, as well. One of the illustrations I like to use to emphasize the importance of using the column oven rather than ambient temperature relates to a couple of trips I took in 2008. In January I was in central China in a laboratory that was not heated, and the ambient temperature was 10 °C; six months later I was in a temporary laboratory in Israel where, if the temperature wasn't 35 °C, it certainly felt like it! These temperatures are drastically different from the 20–25 °C that most of us would quote when asked to put a number on "ambient" temperature. Imagine trying to transfer a method between those two laboratories. In addition to control of the column temperature, it is important that the mobile phase be preheated sufficiently so that there is no more than a maximum of ≈6 °C difference between the column inlet and outlet.
Following this discussion in one of the classes, an attendee asked about the influence of the temperature of the solvent reservoir and of the autosampler. My feeling is that the temperature of the solvent in the reservoir is not important, because by the time the solvent travels through the pump and autosampler and all the associated connecting tubing, any preheating or cooling provided in the reservoir will be canceled out by the thermal mass of the system. The control of the temperature of the autosampler is most commonly accomplished by heating or, more commonly, cooling the sample tray. The goal with autosampler cooling is to prevent sample degradation before injection, and because it only cools the sample it will have little or no influence on the mobile-phase temperature.
So the conclusion of the discussion is that you should always use the column oven to control the column temperature, even if it is just to set it a few degrees above room temperature — for example at 30–35 °C — to ensure a constant column temperature and thus more stable retention times. You should cool the autosampler tray if your samples are likely to degrade at room temperature before they are injected. Heating or cooling the mobile-phase reservoir has little, if any, affect on the chromatography.
Broad Peaks with a New Column
Another class attendee wondered why he observed broad peaks for his sample after installing a new column. This is a good example of a time to apply the divide-and-conquer principle of troubleshooting — do a physical or mental experiment to divide the problem up into smaller parts so that you can eliminate as many possible causes as you can. Before doing anything, I would reinject the sample, and if the peaks were still broad, I would reinject a reference standard. If both of these injections are still bad, it is not a problem with the particular sample.
My first question would be to determine if the problem occurred because the column was replaced. Were the peaks of normal width before column replacement, but broader after? If so, then either the new column is bad, a nonequivalent replacement was used or something happened in connecting the column to cause the problem. The connections at each end of the column may be the source of the problem, especially if polyetheretherketone (PEEK) tubing and fittings are used. If these are not seated properly, a small gap in the connection can result in extracolumn volume and corresponding peak broadening.
When adjusting PEEK fittings, I recommend turning the pump flow off, loosening the fitting, pushing the tubing into the connection to ensure it hits the bottom and then tightening the nut. If you try to tighten the fitting with the flow on, it can slip during the tightening process, creating exactly the problem you are trying to correct. Once again, reinject a reference standard to see if the problem is now corrected. If the problem persists, the next easy step would be to replace the column with another new column, double-checking that the manufacturer and part number is the same as the original column. While it is rare to receive a bad column today, it can happen. No harm is done by double-checking with another new column, and if it is good, you can just put the spare column in stock for later use.
Another check that should be made is to examine the chromatogram (or chromatograms) for other changes. Has the retention time of one or more peaks changed? Has the peak spacing (relative retention) changed? If either of these situations has occurred, it suggests a change in chemistry of the system. We've already eliminated the column as the source of problems through substitution of another new column. The remaining chemical possibilities are the mobile phase and the column temperature. If the problem origination coincided with a change in mobile phase, this is a likely source of the problem. For example, if acids or bases are present, an error in pH adjustment of the mobile phase might be the problem source. Because it is easy to check by substitution, make up a new batch of mobile phase and see if the problem is corrected. The final chemical possibility is a change in column temperature. Make sure the column oven is on and set to the proper temperature.
The remaining items on my list fall in the extracolumn effects category. We've already looked at the column connections, but extracolumn peak broadening can occur because of poor tubing connections wherever the sample contacts, so connections at the autosampler and detector should be checked as well, especially if PEEK tubing and fittings are used. The detector flow cell, time constant (noise filter) and data system data rate also can be sources of peak broadening. I'm assuming that the detector was not changed, so the flow cell is not likely the source. The detector time constant is an electronic filter that helps to reduce the noise through signal averaging, but if it is set to too large a value, it can result in broadened peaks. The rule of thumb is that it should be set to ≈0.1 times the width of the narrowest peak of interest. Similarly, the data system sampling rate should be set so that at least 15–20 data points are collected across the peak. Although neither of these settings is likely to change without intervention by the operator, a power outage or other factor might cause these to be reset to default values that are not appropriate for the present samples. It is easy to check both the time constant and data rate to be sure they aren't the source of the problem.
The final item on the extracolumn effects list is the possibility of the use of too strong of an injection solvent, too large of an injection or both. My rule of thumb is that you can inject ≈15% of the volume of the first peak of interest if you inject in mobile phase. So, for example, if the first peak is 0.1-min wide and the flow-rate is 1 mL/min, the peak volume is 100 µL. This translates to ≈15 µL for an injection volume if mobile phase is used as the injection solvent. If a stronger solvent is used for injection, smaller volumes should be used; if a weaker solvent is injected, larger volumes may be possible. The simplest way to check for injection problems is to inject a very small volume (for example, ≤5 µL) to see if the problem is corrected. I've seen a case where a method worked well for years, then one day started giving broad or split peaks for no apparent reason. On examining the conditions, it was discovered that too much of too strong a solvent was injected. By diluting the injection solvent and increasing the injection volume to maintain the same injection mass, the problem was solved. Unfortunately, too many methods are in use where the operating conditions are "right on the edge" of reliability — it takes only a minor, and often unidentified, change in conditions to cause them to fail.
The preceding discussion followed a specific series of steps to solve the problem. In any particular case, some of these steps could be eliminated by mental experiments or it may be more logical in your case to perform the test steps in a different order. Additional questions might also be appropriate, such as asking if the method ever worked, or if it was being transferred from another lab or a literature method. Do your best to eliminate possible causes by a bit of mental work before you start doing physical experiments to correct the problem.
John W. Dolan is vice president of LC Resources, Walnut Creek, California, USA. He is also a member of LCGC Europe's editorial advisory board. Direct correspondence about this column should go to "LC Troubleshooting", LCGC Europe, 4A Bridgegate Pavilion, Chester Business Park, Wrexham Road, Chester, CH4 9QH, UK, or e-mail the editor, Alasdair Matheson, at amatheson@advanstar.com
References
(1) J.W. Dolan, LCGC Europe 24(11), 592–596 (2011).
(2) L.R. Snyder, J.J. Kirkland and J.W. Dolan, Introduction to Modern Liquid Chromatography, 3rd ed. (Wiley, Hoboken, New Jersey, 2010).

Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
New Study Investigates Optimizing Extra-Column Band Broadening in Micro-flow Capillary LC
March 12th 2025Shimadzu Corporation and Vrije Universiteit Brussel researchers recently investigated how extra-column band broadening (ECBB) can be optimized in micro-flow capillary liquid chromatography.







