Supported Liquid Extraction: The Best-Kept Secret in Sample Preparation
LCGC Europe
This article provides examples of the use of SLE, demonstrates some of its advantages and provides commercial sources for SLE products.
Supported liquid extraction (SLE) has been around for a long time, but surprisingly it is seldom used to replace classical liquid–liquid extraction (LLE). In SLE, the same aqueous phases used in LLE are coated onto an inert diatomaceous earth support, but instead of shaking the two immiscible phases together, the organic phase is passed through the column (or cartridge) and a very efficient extraction takes place. SLE offers many advantages over LLE, including equivalent or more efficient extraction, no emulsion formation, easy automation, less organic solvent use, less labour and less glassware.
Liquid–liquid extraction (LLE) is probably the most widely used sample preparation process. It has been around for many decades, the theory is fairly easily understood and thousands of publications have demonstrated its usefulness. Most chromatographers are first exposed to the technique in their high school or undergraduate chemistry class. I covered the basics of LLE in a previous "Sample Prep Perspectives" instalment (1), and they will not be repeated here.
There are many advantages to the use and application of LLE. First, the operation is fairly simple and the glassware used is inexpensive. Common solvents are often used for extraction, and most applications involve an aqueous phase and an immiscible organic phase. Initially, the sample can be in either phase, and, depending on the partition coefficients of sample and matrix components in the two phases, compounds of interest often can be quantitatively transferred without the presence of interferences. Adjustments to extraction conditions such as changing pH and ionic strength of the aqueous layer or addition of a second organic solvent to the organic layer are easily made. Recoveries can be quite high and reproducible.
On the other hand, there are also some disadvantages to traditional LLE. First, it involves a great deal of time with laborious shaking that can be user dependent. Vigorous shaking (or even non-vigorous shaking) can result in emulsion formation. Emulsions can be difficult to break, and additional time is usually involved in performing this activity. In its traditional application, the technique consumes large amounts of organic solvents and generates considerable waste. Glassware must be cleaned after each use. Automation is very difficult; the technique is performed serially rather than in parallel so throughput is reduced.
Supported Liquid Extraction
An alternative approach that gets around some of these disadvantages has been available for some time yet is relatively unknown — supported liquid extraction (SLE, sometimes referred to as solid-supported liquid extraction or solid–liquid extraction) (2). Its principles are relatively simple: a chemically inert, high-surface-area, highly purified, graded diatomaceous earth serves as a stationary vehicle for the aqueous phase of the LLE experiment. Water very easily adsorbs onto the surface (and is absorbed by the surface) of the diatomaceous earth particles. The dry solid sorbent [Figure 1(a)] is placed into a cartridge, column or well of a 96-well plate, the same devices used for solid-phase extraction (SPE). As shown in Figure 1(a), the aqueous-based sample (for example, diluted plasma or drinking water) is added to the dry sorbent and allowed to wet (disperse by capillary action and absorption) the diatomaceous earth, a process that usually takes only 5–10 min, sometimes a bit longer. Often the aqueous sample is pretreated (that is, the pH is adjusted or an ion-pairing agent or buffer is added) such that the analyte or analytes of interest are in a suitable form to be extracted into an organic solvent, just as is performed in conventional LLE.
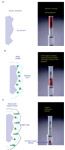
Figure 1: Steps in the supported liquid extraction process: (a) before extraction, (b) apply sample and (c) extract with organic solvent. In (b), allow 10â15 min for the aqueous phase to spread out over the diatomaceous earth. In (c), multiple extractions of small volumes of organic solvent are sometimes more efficient than one extraction with a large volume of solvent.
Next, a small volume of immiscible organic extraction solvent is added to the top of the cartridge and allowed to percolate by gravity (or sometimes with gentle vacuum or pressure) through the supported aqueous phase [Figure 1(c)]. For a more viscous liquid sample, a vacuum is used to pull liquid through the cartridges. Because the aqueous sample has been widely dispersed throughout the solid support, the organic solvent has intimate contact with the thin film of aqueous phase and rapid extraction (equilibration) occurs [Figure 1(c)]. As mentioned above, this intimate contact also occurs in LLE because of the vigorous shaking of the separatory funnel. This shaking causes the immiscible organic solvent to disperse into tiny droplets that provide closer contact to the surrounding aqueous solvent. The downside of this dispersion process in LLE is that emulsions can form and the two phases can take a long time to separate.
The final step in the process is collection of the organic effluent containing the analytes of interest from the outlet of the SLE device [Figure 1(c), right-hand side]. The aqueous phase remains on the device. A phase-separation filter is incorporated into the outlet frit of the device to ensure that organic effluents remain uncontaminated by aqueous matrix.
With SLE, there is no vigorous shaking and therefore emulsions cannot be formed. In addition, the intimate contact between the aqueous and organic phases allows very efficient partitioning and analyte recoveries can sometimes be higher than in conventional LLE. Furthermore, the SLE process is more technique independent than conventional LLE; this often means that the experiments are more reproducible. Obviously, the glassware involved in SLE is greatly reduced compared to that required for LLE and no cumbersome washing of the separatory funnel is required. The entire SLE process is easier to automate than traditional LLE. The 96-well plate SLE format is especially amenable to automation using xyz robotics systems. Prepacked SLE products are available in all automation formats. In addition, bulk sorbent can be purchased for those who wish to make their own customized SLE devices.
Commercial Sources of SLE Products
Because SLE has been around for many years, a number of companies have developed products to serve the needs of scientists who wish to improve or automate their LLEs. Table 1 lists several companies that supply products. Basically, there are three types of products: cartridges (sometimes referred to as columns or tubes); 96-well plates (mainly for automation); and bulk sorbent for those who wish to prepare their own SLE devices. This list represents typical suppliers; any supplier wishing to provide information on its products can supply information to be included in future updates.
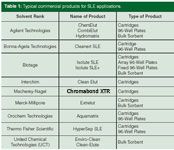
Table 1: Typical commercial products for SLE applications.
Typical Operations in SLE
The SLE approach can duplicate any developed LLE method with a few minor adjustments. In general, 1 g of diatomaceous earth sorbent is needed for each 1 mL of aqueous sample. Cartridge size is determined by the total volume of sample that needs to be extracted. The volume extracted is dependent on the concentration of the analyte or analytes of interest, the total volume of sample available and the sensitivity of the analytical system that will make the downstream measurement. For an environmental sample (for example, for screening pesticides in water), cartridges with an aqueous capacity volume of up to 100 mL are available. For biological samples such as plasma or urine, if one is using liquid chromatography (LC)–mass spectrometry (MS)-MS (triple quadrupole) for analysis, the analysis technique's excellent sensitivity would dictate that a smaller sample volume be used. For example, when using 96-well plates with a 2-mL well volume to extract a plasma sample, as little as 200–400 µL (with a maximum volume of 500 µL) can be used to obtain picogram-per-millilitre sensitivity. For the organic extraction, the volume of organic solvent should be at least equivalent to the volume of aqueous sample. As a rule of thumb, at least two column volumes of organic extraction solvent are recommended for maximum recovery.

Table 2: Solvent mixtures compatible with SLE*.
Almost any nonpolar solvent can be used as the immiscible organic phase. The organic solvent should be spectroscopic grade or better because the collected fraction is often evaporated to dryness, and any solvent containing nonvolatile impurities will be unsuitable for performing clean extractions. Pure organic solvents or organic solvent mixtures can be used. Table 2 provides a list of solvent mixtures compatible with SLE. The column to the right of each solvent pair indicates the maximum percentage of polar solvent tolerated. Exceeding the maximum water-miscible solvent percentage may have a detrimental effect on clean-up because some of the aqueous phase containing impurities could be stripped from the diatomaceous earth.
Applications of SLE
To show the ease of converting an LLE method to an SLE device, I will illustrate two examples, one fairly simple and one more complex. Parabens are chemical compounds used in many processes in the pharmaceutical, cosmetic and food industries. The most commonly used parabens are the methylparaben, ethylparaben, propylparaben and butylparaben analogs, which are sometimes used singly but also in combinations. They are most frequently used as preservatives to prevent microbial growth in the products.
A study of these four parabens was used to compare LLE to SLE (3). A previous SLE method was used as the starting basis (4) and was slightly modified for the paraben standards. For sample pre-preparation, 1 g of body wash lotion was spiked with a mixture of paraben standards; both high (175 µg/mL) and low (20 µg/mL) levels were investigated. Then the mixture was vortexed for 30 s. Next, 2.5 mL of acetone and 1.25 mL of a sodium chloride solution (20 g/100 mL) was added (as in the original LLE method), and the mixture was vortexed again for 30 s. For the experiments, a blank body wash lotion sample as well as a blank of the paraben standards were carried through the extraction process.
LLE Experiment: The prepared sample and blank were each transferred to separatory funnels. A 20-mL portion of ethyl acetate was added followed by 5 mL of sodium chloride solution (20 g/100 mL). The separatory funnel was stoppered and then inverted and shaken with venting several times for 1 min. The phases were allowed to separate and the organic phase was collected. A 0.2-g amount of magnesium sulphate was added to the extract to remove water. The organic solvent was evaporated to dryness and reconstituted in 200 µL of methanol, which is a solvent that is more compatible with reversedphase chromatography. An aliquot of the sample and blank were separately injected into the LC column.
SLE Experiment: The sample and blank were separately loaded onto ChemElut SLE cartridges (Agilent Technologies, 5 mL unbuffered, Part number 1298006) packed with diatomaceous earth. A time period of 15 min was allowed for the sample to disperse into the sorbent. Two 10-mL portions of ethyl acetate were percolated through the cartridge and were collected at the exit. The organic solvent was evaporated to dryness and the residue was reconstituted with 200 µL of methanol, and an aliquot of sample and an aliquot of blank were separately injected into the LC column.

Figure 2: Reversed-phase chromatograms of blank body wash extracts: (a) SLE blank, (b) LLE blank. Column: 150 mm à 4.6 mm, 5-µm Zorbax Eclipse Plus C18; mobile-phase A: water; mobile-phase B: acetonitrile; gradient: 30â65% B in 4 min, then 65â100% B in 1 min; flow-rate: 2.0 mL/min; detection: UV absorbance at 230 nm; injection volume: 1.7 µL.
Results: Figure 2 shows an LC chromatogram of the body wash blank extracted by the SLE method [Figure 2(a)] and the LLE method [Figure 2(b)]. Both techniques showed a substantial number of small extractables that originated in the body wash matrix. For some reason, a large wide peak showed up in the LLE examples at about 5.2 min [Figure 2(b)]. Blanks of the paraben standards carried through the extraction processes showed no impurities of consequence and yielded well-resolved LC peaks (data not shown). Fortunately, the high performance liquid chromatography (HPLC) conditions chosen yielded well-resolved paraben peaks as can be seen in Figure 3, which shows the high-level spike. Recoveries and reproducibilities of the paraben extractions at both high and low levels for LLE and SLE are shown in Table 3. In general, the SLE method gave similar or better recoveries and lower relative standard deviation (RSD) values compared to the LLE method at both levels.

Figure 3: Reversed-phase chromatograms of paraben extracts from body wash high-level spiked sample: (a) SLE extract, (b) LLE extract. Conditions the same as in Figure 2. Peaks: 1 = methylparaben, 2 = ethylparaben, 3 = propylparaben, 4 = butylparaben.
To illustrate a more complex application of SLE compared to LLE, consider the multiresidue confirmation of pesticides in honey (4). This application is very important in the food safety area. Bees that gather pollen from blossoms that have been sprayed with various pesticides can carry the contaminated pollen back to their hives. This contaminated pollen could harm bee populations or lead to the development of contaminated honey. Because bees gather pollen from many different sources, a multiresidue approach is needed to test honey for possible contamination. Pirard's study (4) investigated 17 pesticides and metabolites from a variety of chemical classes. The extraction of pesticides was performed using SLE and compared to the results obtained using traditional LLE.

Table 3: Recoveries of SLE vs. LLE at low- and high-spiked levels of parabens in body wash.
Initially, classical LLE was used to extract pesticides from honey. A 1-g sample of honey was dissolved in 2 mL of water. Next, 6.5 mL of acetonitrile was added and the mixture was mechanically shaken for 30 min. The organic and aqueous layers were separated by centrifugation. The organic layer was evaporated down to 100 µL and added to 100 µL of water. This extract was filtered and injected into an LC–MS-MS system. For the SLE approach, a 1-g sample of honey was spiked with a surrogate standard before mechanical agitation with 1.25 mL of water and 2.5 mL of acetone for 1 h. The mixture was then loaded onto a 5-mL ChemElut SLE cartridge. After waiting for 15 min, the analytes were eluted twice with 10 mL of ethyl acetate (gravity feed). The extract was evaporated to dryness under a gentle stream of nitrogen, and 200 µL was transferred to a 10:90 acetonitrile–water solution in vials for LC–MS-MS analysis. The extracts were analysed by LC–MS-MS in the electrospray ionization (ESI) mode without further purification. The 17 pesticides were separated by optimizing the LC gradient, and the coeluted pesticides with different masses were identified using the multiple reaction monitoring (MRM) mode.

Figure 4: Levels and recoveries of pesticides in honey. Adapted from reference 4.
Figure 4 shows a comparison of recoveries obtained after SLE and classical LLE. The comparison showed that the SLE extraction procedure provided similar or higher extraction efficiency than LLE for most compounds. In particular, note the surprisingly poor recovery results for chlorotoluron, rimsulfuron and 2-hydroxyterbuthylazine for the LLE. For the SLE method, linearity was demonstrated from the range of 0.1–20 ng/g of raw honey with correlation coefficients ranging from 0.921 to 0.999 depending on the pesticide. Recovery rates were well above the range specified in documents (5). Reproducibility was found to be between 8–27%, which was acceptable within the concentrations studied. More than 100 samples of raw honey were analysed to test the method robustness. More details on the method can be found in reference 6.
Conclusions
Although the SLE technique has been around for at least two decades, many separation scientists are unaware of its capabilities and potential to replace traditional LLE procedures. Many sample types have been handled by SLE. The analysis of drugs in biological fluids such as plasma and whole blood is one of the most popular uses of the technique. Generic procedures are available for a wide variety of acidic, neutral and basic compounds. With the use of 96-well plates coupled to LC–MS-MS analysis, only a small amount of sample is required to perform efficient liquid–liquid type extraction in an automated fashion.
Ronald E. Majors is the editor of "Sample Prep Perspectives" and a senior scientist in the Columns and Supplies Division at Agilent Technologies in Wilmington, Delaware, USA. He is also a member of LCGC's editorial advisory board. Direct correspondence about this column should go to LCGC Europe editor, Alasdair Matheson, at Advanstar Communications, 4A Bridgegate Pavilion, Chester Business Park, Wrexham Road, Chester, CH4 9QH, UK, or e-mail amatheson@advanstar.com
References
(1) R.E. Majors, LCGC No. Amer. 14(11), 936–943 (1996).
(2) J.G. Breitenbucher K.L. Arienti and K.J. McClurel, J. Comb. Chem. 3, 528–533 (2001).
(3) H.F. Rossi III, D.W. Keating, D.C. Zimmerman, J. Rizzo and K.M. Usher, Agilent Application Note, in preparation.
(4) C. Pirard, Mass Spectrometry Laboratory, University of Liege, Belgium, published in Agilent Technologies Application Note Si-01002, August 2010.
(5) Commission Decision 2002/657/EC Off. J. Eur. Commun., 12 August 2002.
(6) C. Pirard, J. Widart, B.K. Nguyen, C. Deleuze, L. Heudt, E. Haubruge, E. De Pauw and J.-F. Focant, J. Chromatogr. A 1152(1-2), 116–123 (2007).

Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.
Investigating the Protective Effects of Frankincense Oil on Wound Healing with GC–MS
April 2nd 2025Frankincense essential oil is known for its anti-inflammatory, antioxidant, and therapeutic properties. A recent study investigated the protective effects of the oil in an excision wound model in rats, focusing on oxidative stress reduction, inflammatory cytokine modulation, and caspase-3 regulation; chemical composition of the oil was analyzed using gas chromatography–mass spectrometry (GC–MS).









