Screening of Pollutants in Water Samples and Extracts from Passive Samplers using LC–MS and GC–MS
LCGC Europe
This article describes the GC–MS and LC–MS screening methods developed by the Environment Agency for England and Wales for the analysis of both low-volumn water samples and extracts obtained from various designs of passive samplers.
In December 2000, the European Union's Water Framework Directive (WFD) came into force with the aim of protecting and enhancing the quality of different water bodies within the community. The use of various passive sampling devices in conjunction with gas chromatography–mass spectrometry (GC–MS) and liquid chromatography–mass spectrometry (LC–MS) techniques to screen pollutants has proved invaluable for investigative monitoring purposes within the remit of the directive. Used together, these new analytical approaches offer a robust solution to address specific future monitoring needs, particularly those prompted by legislative change.
The European Union's Water Framework Directive (WFD) became part of law in the United Kingdom in December 2003 with the objective of providing for the planning and delivery of better quality surface water, ground water and coastal waters (1). Various types of monitoring activities are described including: investigative, operational and surveillance. In particular, the WFD will help to deal with diffuse pollution that remains a serious environmental concern after most discharges from point sources have now either ceased or been remediated (2). Furthermore, the Marine Strategy Framework Directive came into force in June 2008, and its key aims are similar; to protect and enhance the environmental status of the marine waters by 2020 (3).

Most of the strategies currently used for the identification of pollutants in a body of water focus on the measurement of the concentrations of the priority substances (currently 33 priority chemicals and eight other pollutants). These are mainly organic compounds together with the metals cadmium, lead, mercury and nickel. The measured concentrations are compared to the proscribed environmental quality standards set by the European Commission for each of these pollutants (4). Each subset of pollutants, such as polyaromatic hydrocarbons (PAHs) or specific classes of pesticides, may require a separate water sample to be taken in the field and the combined cost of these analyses can prove labour intensive and expensive
In such surveillance monitoring campaigns, many compounds that could have a significant toxicological impact on the fauna and flora within the given aquatic environment will remain unidentified. This could result in the environmental objectives for a particular body of water not being met, and the causes of the failure need to be better understood. Further investigative monitoring may then be undertaken to gather further information on the likely reason for the failure. Additionally, within the WFD, the current monitoring practice of taking low volume (1–10 L) bottle, grab or spot samples of water followed by their laboratory analysis may not always provide a useful indication of the environmental status of a water course and alternative approaches, such as biomonitoring, sensors, passive sampling and others may be required.
Existing Approaches to Investigative Monitoring
For several years investigative monitoring within the remit of the WFD has involved the analysis of spot samples of water for unknown (non-target) organic chemical pollutants. This is usually accomplished by low resolution gas chromatographic–mass spectrometric (GC–MS) analytical methods using simple mass spectral library searching routines. GC–MS is a powerful technique for the separation and determination of volatile and semi-volatile compounds, but even with the use of high-resolution capillary columns, it is unable to resolve the multitude of compounds that can be present in complex environmental samples.
Screening of samples via low unit mass resolution GC–MS is also susceptible to interference from other compounds of a similar molecular mass. This implies that by using conventional single quadrupole GC–MS techniques many compounds can remain unidentified, which could have a significant aquatic toxicity (5). It is therefore desirable to introduce other instrumental techniques to improve the quality of environmental assessments and also to benefit from resource reduction as further analytical developments become available (6).
New Analytical Technologies for Environmental Analyses
Several advanced instruments for environmental analyses have been developed recently; including high resolution GC–MS and liquid chromatography–mass spectrometry (LC–MS) systems. These techniques use higher resolution spectrometers such as time-of-flight (TOF), quadrupoletimeof-flight (Q-TOF) and some trap technologies; all have been used effectively to identify complex mixtures of pollutants in water samples (7–11). In addition, nuclear magnetic resonance (NMR) spectroscopy (to confirm structures) in conjunction with high resolution LC–MS has also been proposed (12, 13). Two-dimensional GC (GCGC) has also seen significant advances, especially with the use of fast TOF-MS detectors (14). GCGC allows for enhanced separation of complex mixtures through greater chromatographic peak capacity and allows for the detection of trace contaminants that would not have otherwise been identified through conventional single dimension GC–MS techniques (15).
Multi-target Screening
Many hazardous chemicals have impacts on the aquatic environment at extremely low concentrations and these can be challenging for analytical detection. Complex sample matrices can often make the identification of target compounds very difficult due to significant 'chemical noise', resulting in sub-standard mass spectral library match factors. In order to address these issues, and, in particular the 'chemical challenges' of the WFD, the Environment Agency commissioned the National Laboratory Service (NLS) to develop new low-cost and effective methods to screen pollutants in water samples.
GC–MS screen: The NLS developed the GC–MS target-based multi-residue method (TBMR) which allows for the identification of virtually all GC-amenable pesticides as well as hundreds of other organic pollutants in a single sample. At the heart of the GC–MS screening capability is the de-convolution reporting software (DRS) application for target compound analysis (16). This application combines results from the GC–MS Chemstation (Agilent Technologies, Santa Clara, California, USA), the automated mass spectral de-convolution and identification software (AMDIS — http://chemdata.nist.gov/mass-spc/amdis/) and the mass spectral search program from the National Institute of Standards and Technology (NIST) in a single report. A website that provides an explanation of how AMDIS works can be found at http://chemdata.nist.gov/mass-spc/amdis/explanation.html
Extraction and analytical method: Aqueous samples (including surface water, groundwater and effluent) are collected into 1 L glass bottles and stored in the dark at 5 °C ± 3°C without further additives. As a result of a wide range of compounds contained within the target database and their variety of chemical characteristics, a liquid-liquid extraction method was chosen as the initial isolation method. An internal standard (D10-phenanthrene) is added to a water sample (1 L) which is extracted using dichloromethane (50 mL). The extraction solvent is removed and the remaining aqueous layer acidified (pH ~ 1–2) using sulphuric acid. The extraction procedure is then repeated on the acidified sample. The combined extracts are then carefully evaporated to avoid significant losses of volatile compounds to 1 mL using a nitrogen 'blow down' concentrator. The resultant extract is dried using anhydrous sodium sulphate and transferred to an auto-sampler vial for analysis. Typically, a batch of twentyfour samples can be prepared in a single day. Extracts are analysed using a single quadrupole GC–MS (Agilent 7890-5975 instrument) running in full scan mode (mass range: 35–566 Da) with de-convolution reporting software (DRS) that incorporates mass spectral de-convolution with conventional library searching and quantification. Sample extracts (1.5 µL) are introduced via cold splitless injection and separated on a HP5-MS UI capillary GC column (30 m × 25 µm × 0.25 µm film). (Agilent Technologies, Santa Clara, California, USA). The initial oven temperature of 40 °C (2 min) is increased to 300 °C at 10 °C/min and held for 8 mins. Over 990 target compounds can be analysed by this method without further clean-up. Figure 1 shows a typical chromatogram for a groundwater sample extracted and analysed under the above conditions.
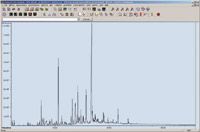
Figure 1: Total ion chromatogram of an extracted 1 L groundwater sample analysed using a GCâMS instrument running in full scan mode (35â566 Da). The extract (1.5 µL) is introduced via cold splitless injection and separated on a HP5-MS UI capillary column (30 m à 25 µm à 0.25 µm film) at the conditions described in the text.
Data analysis: Firstly, the GC–MS Chemstation software performs a quantitative analysis for target compounds using the target ion and up to three qualifying ions under retention time locking (RTL) conditions. RTL has the ability to very closely match chromatographic retention times in any Agilent GC system even if there are subtle differences in columns differing from the nominal length due to 'end trimming' or when there are different column outlet pressures. The DRS software then sends the data file to AMDIS which de-convolutes the component spectra and searches the NLS generated target database using the de-convoluted full spectra (provided peak apices are > half a scan apart). The NLS target database comprises the Agilent RTL Hazardous Industrial Chemicals Database (a mass spectral database of 567 pesticides, solvents and endocrine disrupting compounds), plus a further 423 compounds added by the NLS based on environmental risk assessments. These mass spectra are generated under RTL conditions and entries include the retention times from the locked method. The de-convoluted spectra for all hits identified by AMDIS are sent to the NIST mass spectral library for confirmation. The routine searches against all of the 160000 compounds contained in the NIST library. This is also a full spectrum search, but now against a different library than that used by AMDIS. This approach provides three independent complementary steps of confirmation, thereby significantly increasing the accuracy of target identification, even in the most challenging of matrices.

Table 1: Example of a report from a GCâMS DRS analysis of a contaminated groundwater sample. Compounds identified include the commonly found molluscicide metaldehyde, the herbicide bentazone and the plant growth regulator chlorpropham.
The amounts shown in the quantitation and DRS reports are an estimate of concentration based on target ion response compared to response of the internal standard. This new method has been essential for identifying emerging environmental pollutants. Table 1 shows a report obtained from the GC–MS DRS analysis of an extracted groundwater sample containing several pesticides. The de-convoluted spectrum obtained from AMDIS is searched against the NLS RTL target database and a match factor assigned. This is followed by the retention time comparison to that in the RTL database (R.T. Diff. Sec.). As a final compound verification, the match factor obtained from the independent NIST library is shown together with its rank (Hit Num.) in the top 100 hits. High match factors obtained from the two spectral libraries provides high confidence in the data.
LC–TOF-MS
The NLS have also developed a LC–TOF-MS screening method for the more polar pollutants that are not amenable to direct GC–MS analysis.
Extraction: HLB SPE cartridges (200 mg) (Waters Corporation, Milford, Massachusetts, USA) with an automated extraction system are used. Cartridges are conditioned with methanol (8 mL) followed by de-ionized water (8 mL) and the water sample (500 mL, flow-rate 5 mL/min) is then loaded onto the cartridge. After loading, the cartridge is washed with de-ionized water and the sorbent dried fully with high purity nitrogen. The column is then eluted (10 mL) with dichloromethane:iso-propylalcohol:trifluoroacetic acid (80:20:0.1 v/v/v). The eluate is evaporated to dryness in a vacuum centrifugal evaporator and the residue re-solvated (75 µL) in acetonitrile:methanol (1:1 v/v) and de-ionized water added (425 µL). The sample is vortexed, mixed, filtered, transferred to a screw cap silanized vial and stored (2–4 °C) until analysis. Typically, a batch of twenty samples can be prepared in a single day. The extracts are analysed by LC–MS (Agilent 1200 LC system coupled to a Bruker MicroTOF MS) (Bruker Daltonics GmbH, Bremen, Germany).
LC conditions: The SPE extract (100 µL) is injected onto a Atlantis T3 LC column (Waters Corporation, Milford, Massachusetts, USA) (150 mm × 2.1 mm i.d., 3 µm particle size) held at 45 °C. The mobile phase is 2 mM ammonium formate and 0.01% formic acid in de-ionized water (solvent A) and 2 mM ammonium formate and 0.01% formic acid in methanol (solvent B). The gradient is: 5% B (0 min) with a linear increase to 100% B over 25 min, held for 5 min. Equilibration is 10 min at 5% B.
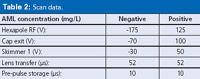
Table 2: Scan data.
TOF conditions: The interfaced TOF-MS is equipped with an electrospray ionization source. The above LC gradient is run twice for each sample: once with the TOF in positive ionization mode and once in negative mode. The nebulizer pressure, dry gas flow, dry gas temperature and capillary voltage are held constant at 50 psig, 10 L/min, 180 °C and 4500 V (negative ionization mode), and 50 psig, 10 L/min, 180 °C and 3000 V (position ionization mode), respectively. The resolution at m/z = 316.962 is approximately 12000. Scan data is acquired across the mass range: 125–1,000 Da at a rate of 1 Hz with the optimized parameters shown in Table 2.
Data analysis: Using the Bruker target analysis software, the TOF data files for each sample are searched for compounds listed in a database. Analysis was performed for the chemicals listed in Table 3.

Table 3: Range of substances analysed in the LCâMS screening method.
Approach: First a calibration is performed to ensure good mass accuracy. Next, extracted ion chromatograms (EICs) are created with a width of ± 0.005 mDa for each compound in the database. The software then looks for peaks in each chromatogram at the retention time specified in the database ± 0.5 min. If a peak is detected, an average mass spectrum (background subtracted) is taken across the peak and the mass and isotope pattern present are scored against the theoretical values. Figure 2 shows an EIC and extracted mass spectrum for the anti-convulsant drug carbamazepine found in a groundwater sample. Analytes were identified using target analysis software (Bruker) which scores compounds in the database against the following criteria:
1. Comparison to known chromatographic retention times,
2. Accurate mass database of environmental contaminants,
3. Isotope patterns for compounds identified in 1 and 2 compared against the theoretical values.
Results for a groundwater sample spiked with a mixture of pesticides, non-ionic surfactants and pharmaceuticals are shown in Figure 3.

Figure 2: Extracted ion chromatogram and mass spectrum for the anti-convulsant drug carbamazepine found in a groundwater sample. The protonated molecular ion for carbamazepine appears in the mass spectrum at m/z=237.1022.
Passive Sampling
The reliability of taking spot samples of water is questionable, as there is a chance that potentially harmful pollutants can be missed if a sample is taken at the wrong time over a pollution event. The 'spot check' approach is only able to collect pollutants present in the column of water the moment the sample is taken. Passive sampling is a technique where pollutants are sequestered by an in-situ device over extended periods of time; typically 1–6 weeks (17). Such devices have been used historically to measure time-weighted averaged concentrations of pollutants in air. This approach increases the likelihood of capturing different pollution events, whether they are point or diffuse. The technique measures the 'toxicologically relevant fraction' of contaminant mixtures and can also indirectly lower analytical detection limits for the various pollutants. Typically, the extracts obtained from passive samplers are chemically complex, especially from those deployed, for example, downstream of a wastewater treatment plant. The analytical challenge is how to cope with the huge number of potential contaminants that may be present in any given sample.

Figure 3: An example of the data output obtained from the analysis of a spiked groundwater. Compounds identified with the symbol +++ meet all three identification criteria; chromatographic retention times and accurate masses match those contained within the data base of environmental contaminants. The isotope patterns also match theoretical values.
Passive samplers
A wide range of passive sampling devices is now available commercially or as laboratory prototypes. A book (18) and a number of reviews (6, 19) are available that describe their construction and use in monitoring the aquatic environment. Two of the main types of sampler used by the NLS in various trials in the United Kingdom are described below.:
Semi-permeable membrane device (SPMD): SPMDs consist of layflat, low-density polyethylene (LDPE) tubing (approximately 98 cm × 3 cm) containing triolein lipid (1 mL) as the absorption matrix (20). SPMDs are used to monitor non-polar organic compounds, defined as those with a log Kow > 3, with maximum cross-sectional diameters of 1 nm and a molecular mass of less than 600 Da. Hydrophobic compounds such as PAHs, polychlorinated biphenyls (PCBs) and organochlorine pesticides in the dissolved state (i.e. those that are readily bio-available) will partition into the SPMD and become concentrated over time (21). Figure 4 shows a SPMD sampler before deployment alongside its protective stainless steel housing. The processing, enrichment and fractionation of SPMDs have been described in a number of publications (22–24) and involve the following steps:
1. Removal of exterior surficial periphyton and debris,
2. Solvent dialysis or extraction using n-hexane,
3. Size-exclusion chromatography and collection of the fraction that contains the chemical classes of interest e.g. PAHs, PCBs and organochlorine pesticides,
4. Class-specific fractionation using adsorption chromatography.
The fraction collected is carefully evaporated to avoid losses of volatile compounds and is reduced in volume to approximately 0.5 mL using a nitrogen blow-down concentrator. The SPMD extracts are analysed by GC–MS using conditions identical to those for the spot water samples.

Table 4 : Different pollutants identified in SPMD and POCIS passive sampling devices deployed in the River Mersey, one of eighteen drinking water protected areas chosen in England and Wales for field trial in 2011. Pollutants identified comprised several WFD priority substances including: the trichlorobenzenes, fluoranthene, benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(a)pyrene, isoproturon and diuron.
Polar organic chemical integrative sampler (POCIS): The POCIS is designed to monitor more polar organic compounds which are not accumulated by SPMDs, i.e. compounds with a log Kow > 3 with maximum cross-sectional diameters of ~ 0.1 µm (25). The POCIS consist of specific SPE resin sandwiched between two polyethersulphone membranes clamped together by steel rings (9 cm outside diameter, 5 cm inside diameter). POCIS are manufactured in-house and the SPE material (200 mg) used is the Oasis HLB phase (Waters). Figure 5 shows a POCIS prior to deployment alongside its protective stainless steel housing.

Figure 4: The SPMD (bottom left) is used to sequester non-polar compounds such as the PAHs, PCBs and organochlorine pesticides (with log Kow > 3), whereas the POCIS (bottom right) adsorbs polar compounds such as pharmaceuticals, some herbicides and fluorinated acids (with log Kow
Extraction of POCIS: The SPE material contained within the POCIS is transferred into an empty 6 mL glass SPE column with a PTFE frit. A further PTFE frit is then placed on top of the material to keep it in place. The SPE column is then eluted (10 mL) with dichloromethane:isopropanol:trifluoroacetic acid (80:20:0.1 v/v/v). This elution mix has been found to give good recoveries for all compounds within the LC–MS database used by the NLS. The evaporation of the eluates and their analyses by LC–TOF-MS is the same as for spot water samples, except that the injection volume is reduced to 10 µL.
Results from the Deployment of Passive Samplers
The NLS has used both designs of passive sampler for a number of field trials in the United Kingdom, and these devices have been used alongside spot water sampling for comparison. The most recent trial involved eighteen protected drinking water areas in England and Wales. Article 7 of the WFD requires member states to put such additional monitoring and associated measures in place to prevent the deterioration of raw water quality so that the need for treatment is reduced (1). Table 4 lists the individual WFD priority substances plus other compounds identified in the SPMD and POCIS from a site on the River Mersey, England. Many of the WFD priority substances identified in the extracts from the SPMDs deployed river have not been identified previously using spot sampling and the GC–MS TBMR screen.
The SPMD can effectively sequester large volumes (10–100 L depending on the analyte of concern) of water over their deployment time. This both permits time integrated sampling that compensates for fluctuating discharges and also gives much lower analytical detection limits. If uptake rates (expressed as the volume of water cleared per unit time for each compound) for the different pollutants are known, time-weighted average concentrations can also be calculated allowing for more realistic mass loadings of a pollutant on a water course to be estimated.
A number of pollutants were also identified by the POCIS and these include the newly classified emerging contaminants such as the fluorinated acids and sulphonates; PFOA, PFHS and PFOS. Various classes of pharmaceuticals including beta blockers (used to treat hypertension), antibiotics, anti-fungals and analgesics were also found. These substances were not identified by spot sampling during the field trial. As a result of a lack of uptake rate data for most of the compounds identified by the POCIS, time-weighted average concentrations could not be determined. Further work in this area is now ongoing. This does not detract, however, from the main objective of the trial which was to identify if passive sampling could produce additional data that would benefit the Agency's investigative monitoring programmes. These trials have shown that passive sampling techniques should have an important role to play in the WFD chemical monitoring requirements. It is possible that the use of these devices will allow for certain water catchments to be discounted while others are targeted and monitored more intensively for WFD purposes.
Conclusions
The new screening methods fill the gaps created by historically reliant risk assessments, and validate the data through a refining of the original risk assessment. Passive sampling together with the GC–MS and LC–MS targetbased screening methods will also identify many emerging contaminants that provide a powerful combined monitoring tool in assessing the pressures on a given water course, helping to ensure that legislation is met. Together these new techniques offer a robust solution to address specific future monitoring needs, particularly those prompted by legislative change such as the WFD.
Anthony Gravell is a technical specialist at the Environment Agency's National Laboratory Service laboratory based in Llanelli, Wales. He specializes in passive sampling in conjunction with HPLC–MS techniques for the analysis of pesticides, pharmaceuticals and endocrine disruptors in various environmental compartments. He is responsible for the development of methods to meet future Environment Agency needs such as the European Union's Water Framework Directive and the Marine Strategy Framework Directive.
Graham Mills is a professor of environmental chemistry (since 2008) at the School of Pharmacy and Biomedical Science, University of Portsmouth, Portsmouth, UK. His research interests include the monitoring of pollutants in water and he is the co-inventor of a novel and low-cost passive sampling device - the Chemcatcher. He has been involved in a number of national and international research projects related to the EU's Water Framework Directive.
Wayne Civil is a technical specialist with the National Laboratory Service of the Environment Agency of England and Wales. His team, based at NLS Starcross, is responsible for the development and implementation of analytical solutions to meet the ever changing environmental monitoring requirements, driven largely by the introduction of the European Union Water Framework Directive.
References
(1) European Commission, The European Water Framework Directive (WFD;2000/60/EC) (http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2000:327:0001:0072:EN:PDF)
(2) C. Stoate, A. Báldi, P. Beja, N.D. Boatman, I. Herzon, A. van Doorn, G.R. de Snoo, L. Rakosy and C. Ramwell, Journal of Environmental Management, 91(1), 22–46 (2009).
(3) European Commission, The European Marine Strategy Framework Directive (MSFD; 2008/56/EC) (http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:164:0019:0040:EN:PDF)
(4) European Commission, Directive on Environmental Quality Standards (Directive 2008/105/EC) (http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32008L0105:EN:PDF)
(5) S. Pedersen-Bjergaard, S.I. Semb, J. Vedde, E.M. Brevik and T. Greibrokk, Chemosphere, 32(6), 1103–1115 (1996).
(6) I.J. Allan, B. Vrana, R. Greenwood, G.A. Mills, B. Roig and C. Gonzalez, Talanta, 69(2), 302–322 (2006).
(7) M. Krauss, H. Singer and J. Hollender, Analytical and Bioanalytical Chemistry, 397(3), 943–951 (2010).
(8) T. Portolés, E. Pitarch, F.J. López, J.V. Sancho and F. Hernández, Journal of Mass Spectrometry, 42(9), 1175–1185 (2007).
(9) I. Bobeldijk, J.P.C. Vissers, G. Kearney, H. Major and J.A. Van Leerdam, Journal of Chromatography A, 929(1–2), 63–74 (2001).
(10) R. Diaz, M. Ibanez, J.V. Sancho and F. Hernandez, Analytical Methods, 4(1), 196–209 (2012).
(11) A. Muller, W. Schulz, W.K.L. Ruck and W.H. Weber, Chemosphere, 85(8), 1211–1219 (2011).
(12) M. Godejohann, L. Heintz, C. Daolio, J.-D. Berset and D. Muff, Environmental Science & Technology, 43(18), 7055–7061 (2009).
(13) M. Godejohann, J.-D. Berset and D. Muff, Journal of Chromatography A, 1218(51), 9202–9209 (2011).
(14) J. Beens and U.A.Th. Brinkman, Analyst, 130(2), 123–127 (2005).
(15) E. Skoczyñska, P. Korytár and J. de Boer, Environmental Science & Technology, 42(17), 6611–6618 (2008).
(16) P. Wylie, M. Szelewski, C.-K. Meng and C. Sandy, Comprehensive Pesticide Screening by GC/MSD using Deconvolution Reporting Software, Agilent Technologies, publication 5989-1157EN.
(17) O. Gangfeng and J. Pawliszyn, Journal of Chromatography A, 1168(1–2), 226–235 (2007).
(18) R. Greenwood, G.A. Mills and B. Vrana (eds.), Passive sampling techniques in environmental monitoring, Comprehensive Analytical Chemistry series, D. Barcelo (series Editor), Elsevier, Amsterdam (May 2007).
(19) B. Vrana, G.A. Mills, R. Greenwood, I.J. Allan, E. Dominiak, K. Svensson, J. Knutsson and G.M. Morrison. Trends in Analytical Chemistry, 24(10), 845–868 (2005).
(20) J.N. Huckins, M.W. Tubergen and G.K. Manuweera, Chemosphere, 20(5), 533–552 (1990).
(21) B. Vrana, A. Paschke, P. Popp and G. Schüürmann, Environmental Science and Pollution Research, 8(1), 27–34 (2001).
(22) J.N. Huckins, G.K. Manuweera, J.D. Petty, D. Mackay and J.A. Lebo, Environmental Science & Technology, 27(12), 2489–2496 (1993).
(23) V. Yusà, A. Pastor and M. de la Guardia, Analytica Chimica Acta, 540(2), 355–366 (2005).
(24) K.-D. Wenzel, B. Vrana, A. Hubert and G.G. Schüürmann, Analytical Chemistry, 76(18), 5503–5509 (2004).
(25) D.A. Alvarez, J.D. Petty, J.N. Huckins, T.L. Jones-Lepp, D.T. Getting and J.P. Goddard, Environmental Toxicology and Chemistry, 23(7), 1640–1648 (2004).

Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.









