Quantifying AAV Quality Attributes Using SEC–MALS
Adeno-associated virus (AAV) is an attractive delivery vehicle for gene therapy because of its mild immune response and ability to deliver the therapeutic gene into a wide range of host cells. To ensure the safety and efficacy of the viral vector-based drug product, it is essential to implement robust and reliable characterization tools throughout research, development, production, and manufacture. This article describes a method to measure three important AAV quality attributes: 1. total number of viral capsid particles; 2. relative capsid content, for example, ratio of full to total capsids; and 3. percentage of aggregates. The method uses size-exclusion chromatography (SEC) coupled to multi-angle light scattering (MALS), UV, and differential refractive index (dRI) detectors. Orthogonal methods based on high-throughput dynamic light scattering (HT-DLS) and field-flow fractionation (FFF) are reviewed and compared.
Over the last forty years, significant strides in capsid and gene optimization have made recombinant adeno-associated virus (AAV) a highly sought-after gene therapy vector, resulting in over 200 clinical trials to date and three marketed drugs (1). As engineering of capsids and transgenes improves transfection efficiency and evasion of neutralizing antibodies, and candidates advance through product and process development, clinical trials, and full production, better analytical tools are required to characterize and quantify these novel therapeutic agents. Light scattering provides unique capabilities for AAV quantitation and characterization in a single precise assay.
Size-exclusion chromatography with multi-angle light scattering (SEC–MALS) is a robust technique that is commonly used in the development and release assays of a large variety of biopharmaceuticals. Combining detection by MALS, UV absorption at 280 nm and 260 nm, and differential refractive index (dRI), SEC–MALS quantifies multiple AAV quality attributes (QAs) simultaneously: total capsid concentration (Cp), full-to-total ratio (Vg/ Cp), and purity (aggregation) (2). SEC–MALS eliminates the need to rely on multiple assays for capsid quantitation and characterization and is suitable for use throughout process and product development. Importantly, SEC–MALS provides significantly improved precision and accuracy compared to other techniques. For example, McIntosh et al. reported determination of total capsid concentration via SEC–MALS with <5% error relative to known values, as compared with SEC-UV (A260/A280 ratio), which resulted in >50% error, depending on the capsid content (Vg/Cp) (3). Similar low precision has been reported by other techniques, such as enzyme-linked-immunosorbent assay (ELISA) and quantitative real-time polymerase chain reaction (qPCR) (3,4).
The enhanced performance of SEC–MALS stems from its absolute biophysical nature. With no reliance on reagents or indirect reporters such as fluorescence, SEC–MALS determines key parameters directly, while adding deep characterization at no additional cost in time or effort. For example, combining MALS with dRI establishes molecular weight, confirming the identity of each eluting peak which might include monomers, oligomers, fragments, or contaminants; this serves as an internal control for data analysis and run-to-run comparisons. Aggregate quantitation by dRI eliminates uncertainties in UV quantitation related to scattering. Combining online UV and dRI further enables precise measurement of extinction coefficients, which are required for accurate analysis of AAV concentration and full-to-total ratio. In order to simplify rapid quantitation in quality control (QC) release assays, deep characterization can be dropped and the workflow is streamlined with analysis by just MALS and UV (260 nm and 280 nm). All of these analyses require no adjustment for different serotypes.
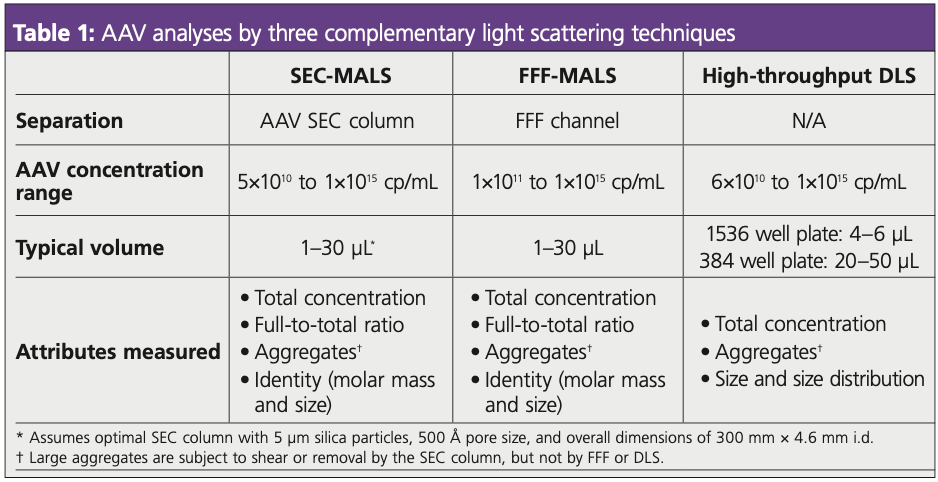
Complementary light scattering techniques, including field-flow fractionation with MALS (FFF-MALS) and high-throughput dynamic light scattering (HT-DLS), enable extended characterization of AAV samples and can be implemented as orthogonal methods to SEC–MALS (Table 1). FFF-MALS enables quantitation and characterization of large aggregates that may be sheared or removed by a SEC column; it is typically used to determine if process changes result in aggregate formation or other impurities (5). HT-DLS measurements rapidly characterize size, low-resolution size distributions, and total capsid count, and help assess thermal and colloidal stability (6).
Experimental
SEC–MALS data were collected using a standard high performance size-exclusion chromatography (HP-SEC) setup and following the procedures laid out in the SOP Guidance Manual: Critical Quality Attributes of AAV by SEC-MALS (Wyatt Technology Corporation) (7). The hardware requirements, sensitivity specifications, and typical method parameters are summarized in Table 1. Briefly, the setup consisted of a high performance liquid chromatography (HPLC) pump, autosampler and diode array detector (Agilent Technologies), plus DAWN MALS detector and Optilab dRI detector (both from Wyatt). MALS, UV, and dRI detectors were plumbed downstream of an SEC column for separation with 5 μm silica particles, 500 Å pore size, and overall dimensions of 300 mm × 4.6 mm i.d. (Wyatt). Pump control and data collection were performed with Astra software while data analysis was carried out with Astra’s Viral Vector Analysis module (Wyatt).
Results and Discussion
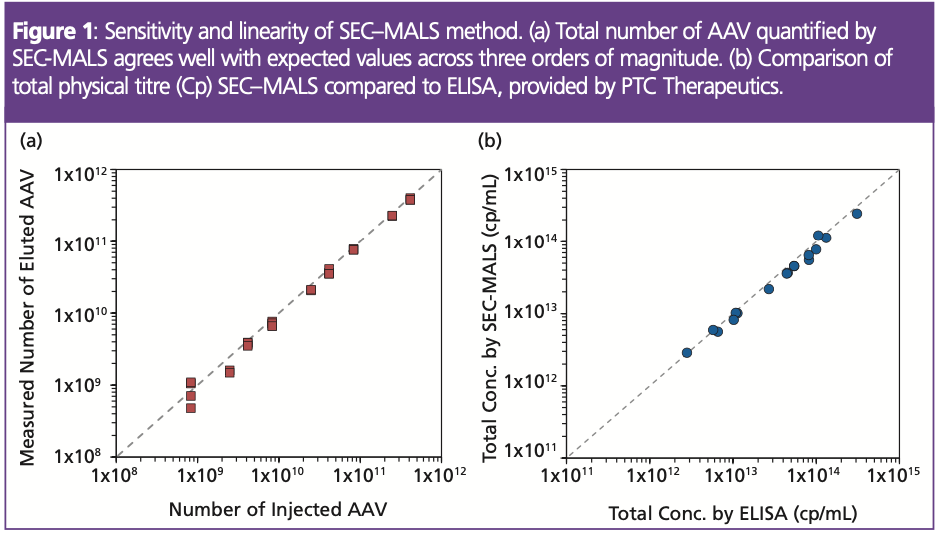
Total Capsid Concentration (Cp): SEC–MALS provides accurate quantitation of the total number of virus particles over a wide variety of concentrations. Run-to-run precision is typically within 5%, regardless of the starting concentration of AAV, and may be as good as 1% under optimal conditions. Figure 1(a) shows typical linearity, accuracy, and precision for capsid concentrations ranging from 8×1010 cp/mL to 8×1013 cp/mL, with injection volumes varying from 1 μL to 50 μL. Equally high precision was observed upon injecting the same total number of capsids, using different combinations of volume and concentration, as for replicate measurements of the same sample. These results compare favourably with more traditional methods, such as ELISA, as shown in Figure 1(b). Considering its automation and lack of complex sample preparation, SEC–MALS is suitable for routine measurements throughout the product and process life cycle.
Full-to-total Ratio (Vg/Cp): Since empty and full AAV have the same hydrodynamic radius, SEC does not separate empty from full viruses; however, separation is not required to quantify full-to-total ratio (Vg/Cp). Rather, data acquired simultaneously from multiple detectors are used to calculate the amount of protein and amount of nucleic acid in each eluting data slice and from these, the ratio Vg/Cp. To generate the data in Figure 2, empty and full AAV controls at a total concentration of 5×1012 particles/mL were mixed together to create Vg/Cp ratios from 0.03 to 0.97. For each mixture, the measured Vg/Cp is within ±0.03 of expected value, representing a level of precision and accuracy comparable to droplet digital PCR (ddPCR) and analytical ultracentrifugation (AUC).
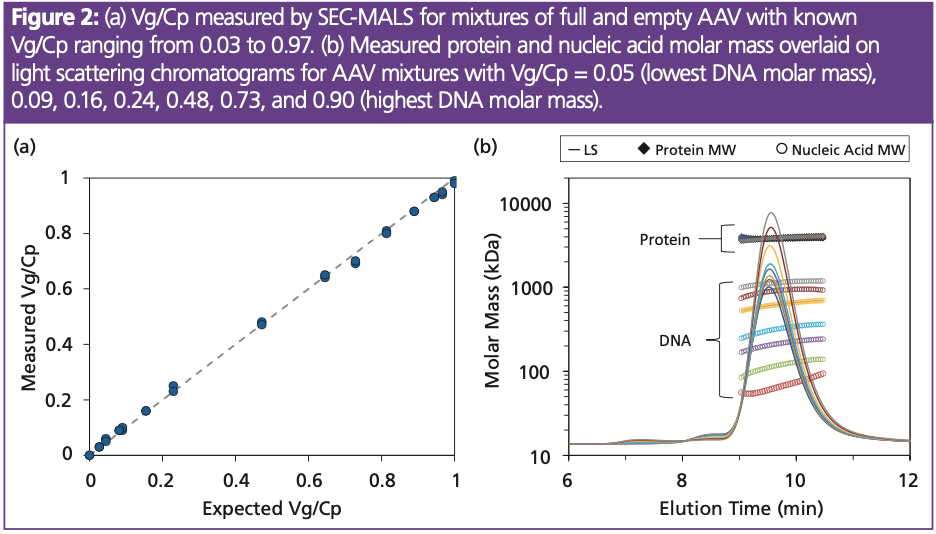
Molar mass measurement by MALS further enhance the Vg/Cp quantitation. As shown in Figure 2(b), SEC–MALS quantifies not only the total molar mass but that of the protein and DNA components. Regardless of Vg/Cp, the protein molar mass measured by MALS reflects the expected capsid molar mass. The measured protein molar mass, therefore, can act as an internal control, ensuring consistent analysis and complete AAV characterization; similar consistency checks are not available with simple SEC–UV measurements. Deviations from the expected capsid molar mass may indicate incorrect assembly, aggregation, fragmentation, or other degradation. These data, combined with physical titre and capsid content, are useful in assessing the consistency of recombinant AAV produced at multiple sites or evaluating cell lines and manufacturing strategies (8).
DNA molar mass measurements can reveal lot-to-lot variability or changes in packing efficiency (Figure 2b). AAV samples with high packing efficiency exhibit measured DNA molar masses at or near the molar mass of the transgene whereas empty AAV result in negligible DNA molar mass. AAV that pack multiple copies of a single transgene (such as in the case of self-complementary DNA) will result in DNA molar mass that is correspondingly larger than expected. By directly measuring the DNA packing, SEC–MALS can provide more accurate quantitation of Vg/Cp than qPCR which may report vast errors in titre in the presence of self-complementary DNA (4).
Aggregation and Extended Characterization: SEC readily separates AAV monomers from oligomers and fragments, and SEC–MALS is essential for characterizing oligomers, aggregates, and other impurities. By measuring the molar mass and radius of each eluting species, SEC–MALS identifies dimers, trimers, larger aggregates, and fragments (Figure 3) and quantifies the relative amount of each species. In conventional SEC–UV, large aggregates may scatter some of the incident UV light, causing overestimation of aggregate content by UV peak area. In contrast, MALS and dRI provide alternative quantitation of large particles and a more accurate counting of these aggregates. SEC–UV–MALS-RI data further identifies peaks that do not contain AAV, such as free DNA released during accelerated stress studies (3).
Complementary, orthogonal light scattering measurements can confirm sample purity and assist in establishing system suitability of the SEC–MALS method. Batch DLS measurements, for example, are rapid, low volume, and nondestructive and may be used both in process development and QC to measure size distribution and to estimate total particle concentration (6). MALS, UV, and RI detection combined with field-flow fractionation (FFF–MALS) provides extensive characterization and quantitation of large species that may be retained or sheared by the SEC column. The combined light scattering toolkit, therefore, provides multiple methods for complete analysis of AAV QAs.
Conclusion
SEC–MALS measures AAV QAs in a single, rapid, automated method, with a high level of precision that analytical SEC or conventional techniques like ELISA and PCR cannot achieve (3,4). Additionally, SEC–MALS does not require special reagents, sample preparation, or adaptation to different serotypes, which allows easy transfer from development into QC and across production sites. Thus, SEC-MALS is becoming an important tool for analytical characterization and quantitation in AAV gene therapy applications.
Acknowledgements
Data comparing measurements of total capsids by SEC-MALS and ELISA were kindly provided by PTC Therapeutics.
References
(1) J.T. Bulcha, Y. Wang, H. Ma, P.W.L. Tai, and G. Gao, Signal Transduct. Target. Ther. 6, 53 (2021).
(2) M. Chen and A. Purchel, “AN1617: Quantifying quality attributes of AAV gene therapy vectors by SEC-UV-MALS-dRI”, https://www.wyatt.com/ library/application-notes/an1617-aav-critical-quality-attribute-analysis-by-sec-mals.html (2019).
(3) N.L. McIntosh, G.Y. Berguig, O.A. Karim, C.L. Cortesio, R. De Angelis, A.A. Khan, D. Gold, J.A. Maga, and V.S. Bhat, Sci. Rep. 11, 3012 (2021).
(4) P. Fagone, J.F. Wright, A.C. Nathwani, A.W. Nienhuis, A.M. Davidoff, and J.T. Gray, Hum. Gene Ther. Methods 23(1), 1–7 (2012).
(5) C. Deng, “AN2003: Quantifying AAV aggregation and quality attributes by FFF-MALS”, https:// www.wyatt.com/library/application-notes/ an2003-quantifying-aav-aggregation-and-quality-attributes-by-fff-mals.html (2020).
(6) X. Zhang, W. Wang, and S. Kenrick, “AN5007: Characterization of AAV-based viral vectors by DynaPro DLS/SLS instruments”, https://www.wyatt.com/library/application-notes/an5007-aav-quantitation-and-stability-analysis-by-batch-dls. html (2019).
(7) Wyatt Technology Corporation, “SOP Guidance Manual: Critical Quality Attributes of AAV by SEC-MALS” (2020).
(8) N. Selvaraj, C.-K. Wang, B. Bowser, T.L. Broadt, S. Shaban, J. Burns, et al., Hum. Gene Ther. Methods PMID: 33397196 (2021). doi: 10.1089/hum.2020.054.
Sophia Kenrick is Director of Analytical Sciences at Wyatt Technology Corporation. She received her Ph.D. in Chemical Engineering from the University of California, Santa Barbara, USA, where she utilized a variety of biophysical techniques, including surface plasmon resonance and quantitative flow cytometry, for characterizing combinatorial protein-binding ligands. Kenrick joined Wyatt in 2010 and has provided support to the Sales and Marketing teams and R&D product development efforts. She supports multiple applications for Wyatt instrumentation, especially in the field of molecular recognition and biomolecular interactions.
Anatolii Purchel joined Wyatt Technology Corporation in February 2019 after obtaining his Ph.D. degree in polymer science from the University of Minnesota, USA, with a focus on developing polymers for oral drug delivery. As an Application Scientist at Wyatt, Purchel is tasked with supporting product sales by analyzing samples for proof-of-concept data, providing on-site or virtual seminars, and working with customers to determine how to best meet their analytical needs.
Michelle Chen is the Vice President of Analytical Sciences at Wyatt Technology Corporation located in Santa Barbara, California, USA. She obtained her Ph.D. from the Chemical Engineering Department at Yale University, USA, where she focused on developing novel HPLC approaches for high-speed and high-efficiency separations of biopolymers. Since joining Wyatt, Dr. Chen has incorporated multi-angle light scattering and dynamic light scattering detection with HPLC and field-flow fractionation, for analyzing synthetic and biological polymers, proteins and nanoparticles. In recent years she has led the Analytical Sciences team at Wyatt Technology to develop novel methods for biophysical characterization of emerging bio-nanoparticles including viral vectors, lipid nanoparticles, and extracellular vesicles.
E-mail: mchen@wyatt.com
Website: www.wyatt.com

Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.
Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.
Rethinking Chromatography Workflows with AI and Machine Learning
April 1st 2025Interest in applying artificial intelligence (AI) and machine learning (ML) to chromatography is greater than ever. In this article, we discuss data-related barriers to accomplishing this goal and how rethinking chromatography data systems can overcome them.
Influence of Concentration in Conventional GPC/SEC and Advanced Detection GPC/SEC
March 21st 2025Sample concentration is a parameter that can influence the quality of gel permeation chromatography/size-exclusion chromatography (GPC/SEC) separations and the obtained results. Understanding this influence can help to support the development of reliable GPC/SEC methods.