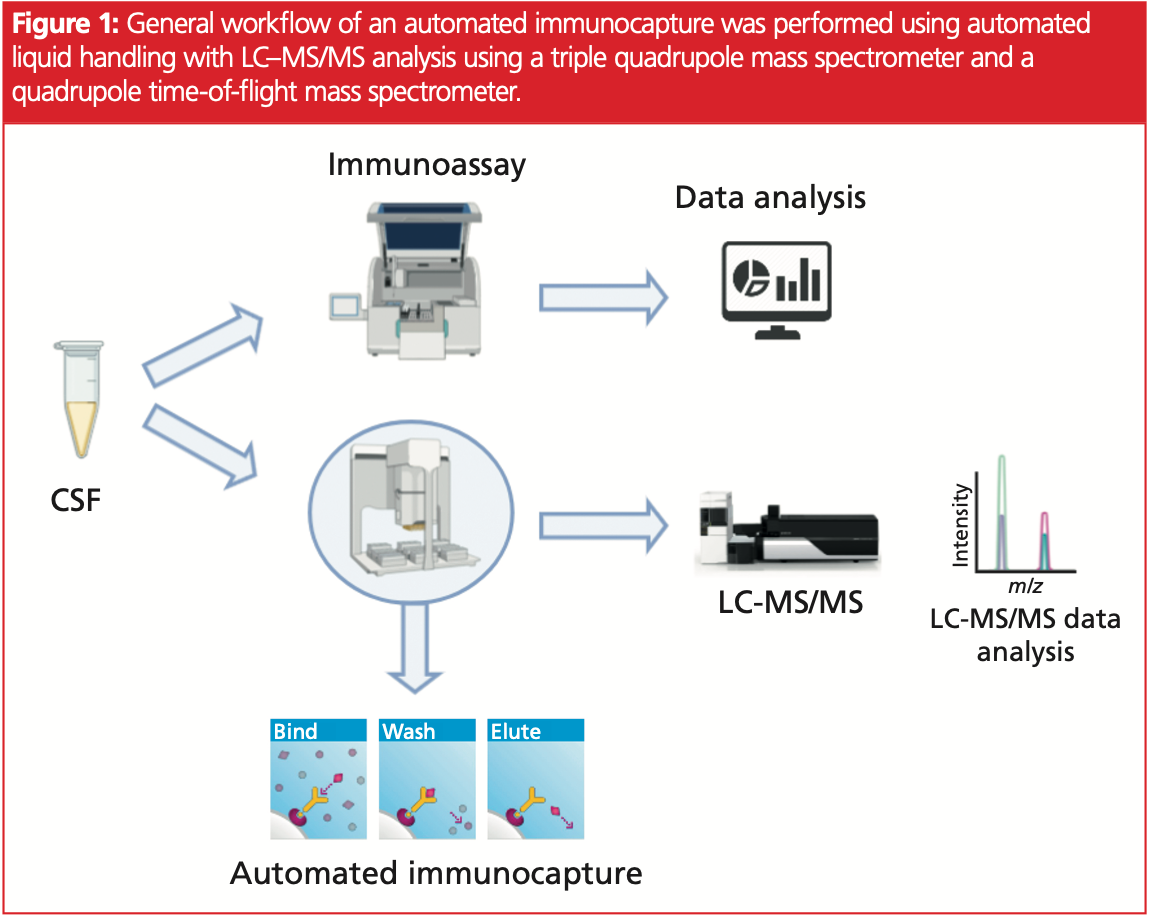
LC–MS/MS for the Quantification of Tau and Phosphorylated Tau in Alzheimer’s Disease Patients’ CSF
Quantification of tau proteoforms in cerebrospinal fluid (CSF) could be useful to help diagnose Alzheimer’s disease (AD), other neurodegenerative diseases, and brain damage. In this article, LC–MS/MS results for tau and phosphorylated tau (pTau) are compared with in vitro diagnostic (IVD)‐qualified immunoassay kits to distinguish between AD patients and control patients (1).
Cerebrospinal fluid (CSF) is a colourless biological fluid directly in contact with the brain. The analysis of CSF proteins is a potential indicator of abnormal states of the central nervous system such as inflammation, infection, neurodegeneration, and tumour growth (2–4). While CSF protein monitoring is useful for diagnostic and prognostic purposes, it also provides valuable information about neurodegenerative diseases’ pathophysiologies.
Tau protein is mainly expressed in the central nervous system and as microtubule‐associated protein, the role of which is to stabilize tubulin polymerization, an essential mechanism for efficient intracellular transport and axonal growth. In the adult brain, 6 tau protein isoforms are translated from a single gene located on chromosome 17, and their expression is regulated by an alternative splicing mechanism. Moreover, tau proteoforms are highly phosphorylated, with about 80 putative sites scattered within the entire protein (5) and notably at position 181.
In Alzheimer’s disease (AD), this hyperphosphorylation will promote its aggregation and thus the formation of neurofibrillary tangles in the brain, which are one of the histological hallmarks of the disease (6). Incidentally, tau is released in the CSF following cell damage, through secretion pathways which still needed to be fully characterized.
Importantly, AD is associated with an increase in tau and phosphorylated tau (pTau) (Serine 181) levels in CSF (7) and their quantification is included in the diagnostic criteria for AD (8). The use of tau and pTau increases diagnostic precision in cases of atypical AD states and helps in refining differential diagnoses. Furthermore, these biomarkers seem to have a predictive value on cognitive decline and disease prognosis (9). Their clinical use is implemented in many clinical laboratories (10) which used in vitro diagnostic (IVD) immunoassays qualified kits that are conformed to the requirements of the clinical norm ISO15189.
The Clinical Proteomics Platform of Montpellier, France developed two complementary mass spectrometry (MS) approaches: a quantitative shotgun method using liquid chromatography coupled to high resolution mass spectrometry (LC‐HRMS), and a quantitative targeted method using triple quadrupole mass spectrometry in multiple reaction monitoring mode (LC‐MRM).
In this work, the scientific team used MS approaches (LC‐MRM and LC‐HRMS) and compared with IVD immunoassay results for CSF tau and pTau residue quantification.
Method
Human samples: CSF samples were obtained from patients, with follow‐up by the Montpellier Neurological and Clinical Research Memory Centers (CMRR) for cognitive or behavioural disorders. Patients gave their informed consent for research and for the storage of their sample in an officially registered biological collection (#DC‐2008‐417) of the certified NFS 96‐900 biobank of the CHRU University Hospital of Montpellier (Ref: BB‐0033‐00031, www.biobanques.eu) The cohort consisted of 19 control patients (CTRL) and 49 patients with AD.
Enzyme‐linked immunosorbent assay (ELISA) tests: CSF tau and tau phosphorylated at Thr181 (pTau) were quantified using IVD commercially available Innotest sandwich ELISA assays (Innotest hTAU ag, Innotest_Phospho‐Tau [181P], Fujirebio) according to the manufacturer’s instructions.
Standards and controls: 15N recombinant tau protein (441) was obtained from Dr. Guy Lippens (UMR8525, Lille Pasteur Institute, France). Lyophilized standards were resuspended at 1 mg/mL with ammonium bicarbonate 50 mmol/L. The solution was then aliquoted into 50 μL in LoBind tubes and stored at −80 °C until use. Standards were diluted with 50 mmol/L ammonium bicarbonate and 1 mmol/L BSA. Human CSF samples with tau concentrations determined by Innotest sandwich ELISA assays were mixed to obtain 3 CSF pool with low tau concentration (LTC, t‐tau around 193 pg/ml), medium tau concentration (MTC, t‐tau around 448 pg/ ml) and high tau concentration (HTC, t‐tau around 615 pg/ml).
CSF tau immunoprecipitation and digestion: After thawing, 250 μL of CSF were incubated with 250 μL of incubation buffer (40 mM Tris) (Trisbase, Sigma Aldrich) at pH 7.4, 137 mM sodium chloride (Sigma Aldrich), 2 mM ethylenediaminetetraacetic acid (EDTA) (VWR), 10 mM Guanidine (Sigma Aldrich) and 2% NP‐40 (Sigma Aldrich)
and with 20 ng of 15N‐tau‐441 (1 μg/ml) and 2.5 μg of biotinylated anti‐tau HT7 antibodies (100 μg/mL) at 4 °C with overnight shaking. Tau immunocapture was performed using AssayMAP Bravo platform (Agilent Technologies) and AssayMAP streptavidin cartridges. The sample was eluted with 25 μL of elution buffer (12 mM NaCl/100 mM HCl, pH 2), neutralized with 20 μL of 100‐mM Tris buffer at pH 8.5 and digested with 1 M of urea, 10% of acetonitrile + 0.5 μg of Trypsine‐LysC mixture for 6 h at 37 °C. After the desalting step, samples were transferred to LC–MS polypropylene vials and frozen at −80 °C before analysis.
LC–MS/MS acquisition: Two complementary approaches of LC–MS/MS analyses were carried out (LC–MRM and LC–HRMS) and Skyline 19.1.0.193 version was used to conduct data treatment. Data was acquired with both methods in the positive ion mode.
LC–MRM: Sample separation was performed using micro‐liquid chromatography (μLC) with a Zorbax high performance liquid chromatography (HPLC) column SB‐Aq column, 1.0 mm × 150 mm, 3.5‐μm (Agilent Technologies). MS analysis of tau peptides was carried out on a triple quadrupole system (LCMS‐8060, Shimadzu Corporation), equipped with an electrospray ionization (ESI) source. The applied mobile phase B gradient increased from 0 to 20% in 51 min, from 20 to 90% in 1 min and from 90 to 0% in 1 min, and 0% up to 60 min. The total acquisition time is 60 min.
LC–HRMS: Sample separation was performed using nano‐liquid chromatography (nLC; nanoElute, Bruker) with a nanoElute 40 cm column, C18X75IDX 1.9 μm (Bruker) (Acclaim PepMap column). MS analysis of tau peptides was carried out on a quadrupole time‐of‐flight (QTOF) system (Impact IITM; Bruker), equipped with an ESI source. The applied mobile phase B gradient increases from 2 to 11% in 55 min, from 11 to 20% in 35 min, from 20 to 95% in 10 min, and 95% up to 110 min. The total acquisition time is 110 min.
Data processing: Skyline (64 bit) 19.1.0.193 version was used to conduct
the data treatment. Based on an identified peptide list, coelution with N15 labelled peptides or aqua peptides, and retention time, masses were extracted from raw data obtained from profile analysis.
Statistical analysis: Descriptive analysis, statistical tests and graphs were performed using Excel software (Microsoft). Statistical tests were performed on RStudio (v1.1.456). Normality was controlled with Shapiro‐Wilk test on log transform data. Analysis of variance (ANOVA) was conducted to compare CTRL and Alzheimer groups with MS and immunoassays results.
Method validation: Three CSF pools with low (193 pg/ml), medium (448 pg/ml),
and high (615 pg/ml) tau concentration with 15N recombinant tau protein (441) internal standard were used to evaluate inter‐day variability, intra‐day variability, and to perform calibration curves. Intra‐day variability was evaluated by an analysis of six replicates of each quality control sample, and inter‐day variability was evaluated over five different batches required to analyze the entire sample pool hailing from the cohort.
Conclusion
Fully automated LC–MRM and LC–HRMS workflows were set up and performances were compared to IVD immunoassay qualified kits (Figure 1).
Mass spectrometry allowed the quantification of several tau protein peptides, giving access to molecular mechanisms occurring in the full‐length protein such as truncations or post‐translational modifications (PTMs). Monitored tau peptides are located between D24 and K223 on the tau protein sequence (tau 441).
Calibration performances were established based on different levels of tau protein in CSF. Coefficient of variation (CV) were between 7–41% depending on the peptide. The R‐squared on the calibration curves were between 0.48 and 0.73.
Obtained concentrations were correlated between ELISA and MS (GAAPPGQK) to tau protein with a Pearson coefficient of 0.8803, and a coefficient of 0.7595 to pTau (T181). Graphical comparison was performed on box‐plot (Figure 2). Statistical differences were observed between AD and CTRL patients.
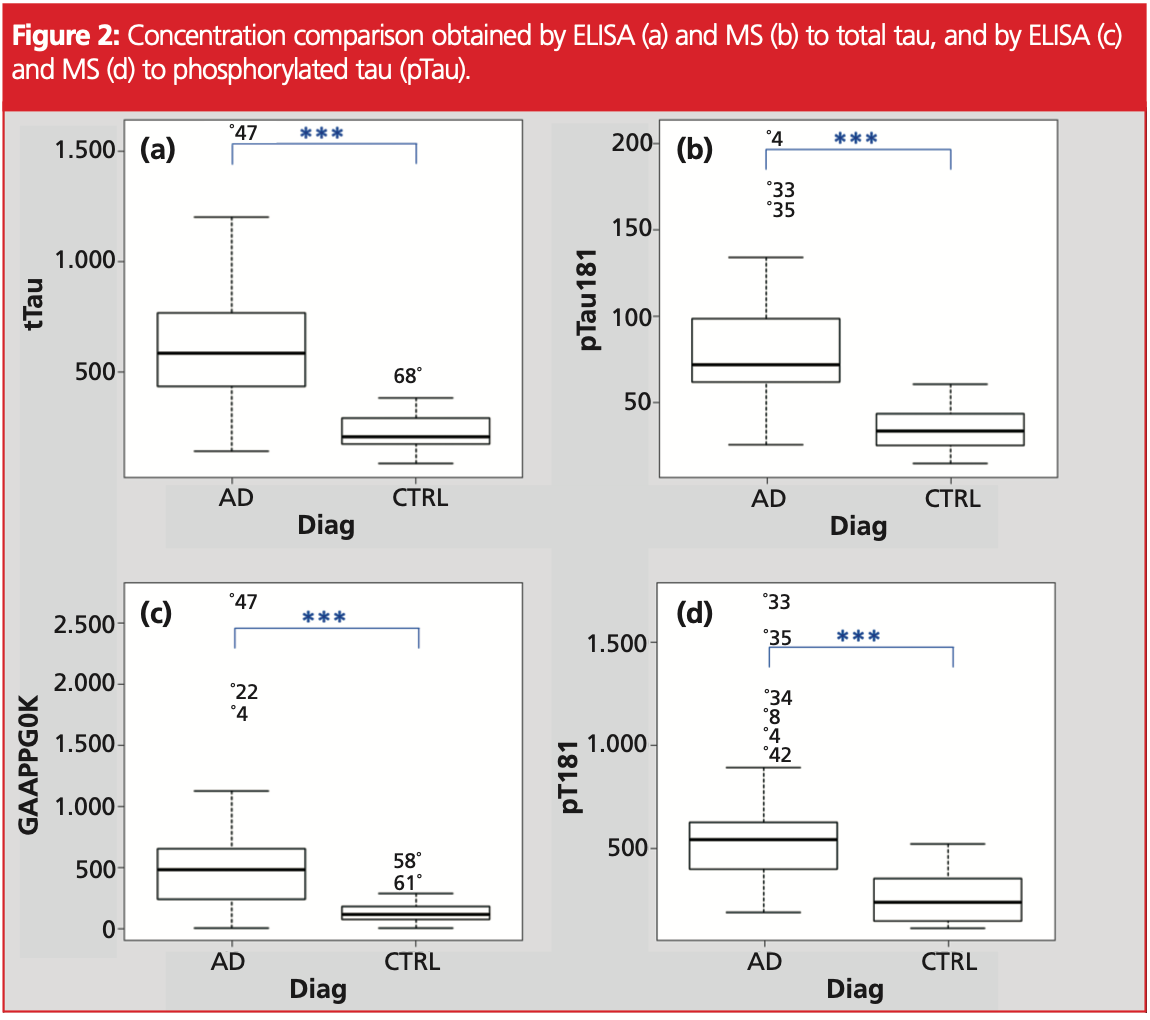
A one‐way ANOVA between disease groups was conducted to compare the capacity to separate CTRL and Alzheimer groups with MS and ELISA measures of tau and pTau.
All the results allowed, to a significant degree, to separate disease groups with a p‐value <0.05 level [F(1, 66) = 53.14, p = 4.94E‐10]. Post hoc comparisons using the Welch test indicated that all the parameters were statistically relevant to separate control and Alzheimer groups (Figure 2).
ELISA and MS techniques were able to discriminate AD and CTRL patients based on pTau or tau with closed performances.
To conclude, ELISA IVD qualified kits and MS analysis gave close performances to quantify tau and pTau, both allowing to distinguish AD patients from CTRL patients. However, LC–MS/MS results have the additional advantage of being able to monitor multiple peptides and phospho‐peptides along the protein sequence, which allows monitoring of specific molecular mechanisms impacting this protein during disease evolution.
Currently, the scientific team is developing the same LC–MS/MS approaches in human plasma (less invasive) to avoid CSF collection and consequently increase the accessibility of these tests. The hope is that such tests will allow, in a clinical context, a more precise follow‐up of AD biomarkers, and provide a valuable clinical tool for prevention and personalized medicine.
References
1. N.R. Barthélemy, Y. Li, N. Joseph‐Mathurin, B.A. Gordon, J. Hassenstab, T.L.S. Benzinger, et al., Nat. Med. 26(3), 398–407 (2020).
2. A.F. Hühmer, R.G. Biringer, H. Amato, A.N. Fonteh, and M.G. Harrington, Dis. Markers. 22(1–2), 3–26 (2006).
3. S. Roche, A. Gabelle, and S. Lehmann, Proteomics Clin. Appl. 2(3), 428–36 (2008).
4. S. Lehmann, S. Schraen, I. Quadrio, C. Paquet, S. Bombois, C. Delab, et al. Alzheimer’s Dement. 10(S5), S390–S4 e2 (2014)
5. K. Iqbal, M. Flory, S. Khatoon, H. Soininen, T. Pirttila, M. Lehtovirta, et al. Ann. Neurol. 58(5), 748–57 (2005).
6. J.‐Z. Wang, Y.‐Y. Xia, I. Grundke‐Iqbal, and K. Iqbal, J. Alzheimer’s Dis. 33(S1), S123–39 (2013).
7. M. Sjögren, L. Minthon, P. Davidsson, A.K. Granérus, A. Clarberg, H. Vanderstichele, et al., J. Neural. Transm. (Vienna) 107(5), 563–79 (2000).
8. G.M. McKhann, D.S. Knopman, H. Chertkow, B.T. Hyman, C.R. Jack Jr, C.H. Kawas, et al. Alzheimer’s Dement. 7(3), 263–9 (2011).
9. N. Mattsson, H. Zetterberg, O. Hansson, N. Andreasen, L. Parnetti, M. Jonsson, et al. Jama. 302(4), 385–93 (2009).
10. A. Gabelle, J. Dumurgier, O. Vercruysse, C. Paquet, S. Bombois, J.L. Laplanche, et al. J. Alzheimer’s Dis. 34(1), 297–305 (2013).
Christophe Hirtz is a fully tenured Professor in Biochemistry and Clinical Proteomics and specializes in clinical assays and R&D activities in the field of clinical proteomics (87 publications, ISI h‐index 24). Hirtz heads the Clinical Proteomics Platform, aiming to exploit the latest technological developments in MS and immunoassay for the discovery, validation, and use of biomarkers in various human pathologies (proteins, RNA, DNA, metabolites). He is involved in multiple scientific projects such as PI (France Alzheimer 2020) and work package leader (ANR SILK road, Proteocovid 2020).
Sylvain Lehmann was trained as an M.D. (1991, Strasbourg University, School of Medicine, France) and gained a Ph.D. in Neurobiology in 1992. He was the recipient of a Howard Hughes fellowship for physicians and spent four years in Washington University, USA, as a postdoctoral fellow and a research assistant professor (1993–1996). There he started working on Prion and AD using cell culture and cell biological tools. From 1997–2002 he was an INSERM Senior Researcher and in 2003 obtained a position of professor of Pathology at the Medical School of Montpellier, France. As a Qualified Medical Biologist/Pathologist from The French National Medical Council (CNOM), his clinical unit at the University Hospital Center (CHU) of Montpellier oversees the biological diagnosis of neurodegenerative disorders and he recently set up a reference Neurological biobank. Lehmann’s laboratory hosts the “Clinical Proteomics Platform” of the CHU and is involved in innovative programmes on biomarker discovery and detection using mass spectrometry and immunoassays. In January 2021, he became Director of the Institute for Neurosciences of Montpellier (INM).
Laura Fichter has been an engineer specializing in mass spectrometry and proteomics at the clinical proteomics platform, since 2019, following the completion of a Master’s degree in Biotechnologies. She is in charge of proteomics projects on the detection and characterization of tau proteoforms and is also involved in various projects on protein analysis in mass spectrometry, including characterization of the Spike protein of SARS‐CoV‐2.
Stephane Moreau obtained his diploma from INSA in fine chemistry and engineering with a specialization in chemical process engineering, in 1994. He then started his professional career in laboratory equipment distribution before joining the brand new Shimadzu France subsidiary in 2002. Since then, Moreau has held various positions to develop the MS range business. Since September 2013, he has been product manager for the MS range with Shimadzu Europe.
Jerome Vialaret is an expert in proteomic, holding positions at Pierre Fabre Laboratories (Oncology center, France), EPFL (Lausanne, Switzerland), INRA (Montpellier, France), and Montpellier hospital, France. He is the team leader of the clinical proteomics platform (https://ppc‐ montpellier.com/index.php/accueilen/) including the technical part, the scientific communications, and the team management. Vialaret has great experience in large‐scale proteomics (proteome, protein‐complex, phosphoproteome, and other PTMs) with dedicated quantitative methods (silac and label‐free). In 2011 he began clinical chemistry development on protein quantification using targeted mass spectrometry in a clinical environment.
E-mail: shimadzu@shimadzu.eu
Website: www.shimadzu.eu

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Advances in Non-Targeted Analysis for PFAS in Environmental Matrices
March 27th 2025David Megson from Manchester Metropolitan University in Manchester, UK, spoke to LCGC International about the latest developments in non-targeted analysis (NTA) of per- and polyfluoroalkyl substances (PFAS) in environmental matrices based on a recent systematic review paper he has collaboratively published (1).