Practical and Popular Sample Preparation Techniques for Ion Chromatography
Special Issues
The authors present the most common and fundamental techniques that address common matrix issues and discuss the critical chemistry considerations.
Techniques that could be included in a discussion of sample preparation for ion chromatography are as diverse as the panorama of sample types containing charged or ionizable targets. In this article, we present the most common and fundamental techniques that address common matrix issues, and discuss the critical chemistry considerations. We show the expected improved data quality by using sample preparation.
Ion chromatography (IC) is first and foremost a chromatographic analytical technique used for the analysis of ionizable as well as permanently ionized target analytes. Components of the sample can interfere directly with the targets or with the eluent components, thus altering the elution behavior. They also can foul the analytical column or compromise analyte detection. The interferents can be charged or neutral, so knowledge of the chemistries involved is important to minimize time spent optimizing a method. The interferents can be identified (known) or be unknowns characterized by their behaviors. Many of the most common application problems in IC and their sample preparation solutions have been described (1,2).
Have the Right Toolbox of Columns
The goal of sample preparation, in any form, is to achieve good quantification, reproducibility, accuracy, and long system life. Sample preparation is a cost component in an analysis and is used because data quality or system ruggedness can be unacceptable without it. The good news is that there are quite a number of separation columns available for IC, each developed to meet a key analytical need. These columns may have higher capacity or optimized selectivity, or both. For some samples, choosing the right column that can separate analytes of interest from matrix interferences can eliminate the need for sample preparation. Likewise, some detection principles such as inductively coupled plasma–mass spectrometry (ICP-MS) can eliminate or minimize sample preparation through the technique's inherent selectivity.
The capabilities available in a well stocked "toolbox" of columns can be invaluable in time and money savings. For example there are a number of "high-low" applications where ratios as high as 10,000:1 sodium:ammonium can be managed isocratically simply by choosing the separation column designed for the analysis (3). In such a case, when the best column is selected for the job, there is no additional cost for sample preparation. As most any tradesperson will say, having the right tool for the job makes all the difference.
Added concepts that expand the column selection option are the use of heart-cutting and two-dimensional heart-cutting. Heart-cutting takes advantage of column selectivity to direct only the targets to a detector such as a mass spectrometer. Two-dimensional heart-cutting combines (usually) two column formats of two orthogonal chemistries to eliminate the matrix while also concentrating the target analytes in one method (4).
The present discussion picks up at that point: What can one do when the need is beyond column selection?
The most commonly used sample preparation technique for IC other than filtration is matrix elimination, with and without off-line or in-line concentration. In this article we review two of the most commonly used techniques for sample preparation in IC and discuss the various formats of their use. Exemplary applications are used to illustrate the key concepts of data quality and ruggedness.
Sample Preparation Phase Chemistries
In IC, the most common sample preparation concept (after filtration) is matrix elimination, which is the concept of selectively removing matrix species while flowing the target analytes into a vial or to a concentrator column. The sample preparation chemistries take advantage of differences in chemical properties between target species and matrix species to accomplish matrix removal. The stationary phase of the sample preparation device is designed for maximally selective retention for the matrix species while exhibiting no retention for the species passing through the resin bed. Table I includes the selective retention principles for sample preparation phases used in ion chromatography. The most common format is an off-line cartridge with Luer connectors for use with a syringe or a vacuum manifold. The technique using this format is often referred to as solid-phase extraction, in this case, of the matrix. Various stationary phases are chosen to extract ionic and organic compounds while letting target components pass through the cartridge. After the samples are "prepared" they can be analyzed by a variety of techniques including IC, high performance liquid chromatography (HPLC), ICP, gas chromatography (GC), nuclear magnetic resonance (NMR) spectroscopy, and hyphenated techniques such as IC–MS and others.

Table I: Cartridge-type sample preparation products
Matrix elimination products are available in several formats that allow off-line or in-line matrix removal of species including chloride (halides), sulfate, azo dyes, fats, transition metals (by several different selectivity phases), common anions, humic acids (polyphenolics), general neutral hydrophobic species, and others. These devices can be used alone or in combination and can have other uses, including pH adjustment. Most of the available chemistries were developed to solve specific application problems, such as the determination of nitrate in a 1.6% NaCl brine solution or the removal of fat from milk to eliminate detector electrode fouling. Table I is a summary of the commercially available chemistries and the off-line products that use them.
Practical Calculations
Use of matrix elimination requires a few calculations to ensure that the device can treat the required sample, in terms of concentration and volume. The overall goal is to determine if the sample preparation cartridge has enough capacity to treat the required volume of sample so that the matrix is actually removed from the sample. Following is an example of a possible sample:
Given: sample contains 1% NaCl as matrix. The target is nitrite, at about 1 mg/L.
Issue: Nitrite is eluted close to chloride, so at high concentration, the chloride peak swamps the nitrite peak, reducing its recovery and rendering quantification impossible.
Solution: Nitrite does not precipitate with silver. A silver-form resin cartridge, such as the OnGuard II Ag cartridge (Thermo Scientific), can remove chloride from a sample matrix by precipitation of silver chloride in the resin bed.
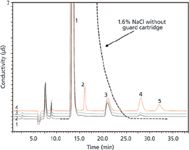
Figure 1: Determination of nitrite in brine with the aid of matrix elimination sample preparation. Column: IonPac AG/AS15, 4 mm; eluent: 23 mM KOH; flow rate: 1 mL/min; sample preparation: InGuard Ag and Na; injection volume: 100 µL; detection: suppressed conductivity, ASRS 300. Chromatogram 1: 1.6% NaCl brine after sample preparation; chromatogram 2: water blank; chromatogram 3: standard of 2 ppm nitrite, sulfate, nitrate in 1.6% brine after sample preparation. Peaks: 1 = chloride, 2 = nitrite, 3 = carbonate, 4 = nitrate, 5 = sulfate.
Calculations: 1% NaCl is 1 g per 100 g or 10 g/L NaCl. The chloride portion of this is 35.45/58.45, 6.06 g/L, or about 0.17 mEq/mL. One OnGuard II Ag cartridge (1-cc) contains about 2.5 mEq of capacity. Dividing the cartridge capacity by the number of milliequivalents per milliliter of the sample, one can theoretically treat about 14 mL of 1% NaCl. The cleanest sample is generally obtained by using about 20% less than the maximum capacity. A white precipitate is visible in the resin bed and can guide the analyst in the determination of breakthrough volume. This cartridge is used in series with a sodium-form resin cartridge (such as the OnGuard II Na cartridge, Thermo Scientific) so that any silver ion from dissolved AgCl can be trapped on the sodium-form resin without any pH change. As a comparison, if the silver-form resin is followed by a hydrogen-form resin, then any trapped silver ion displaces hydronium ion, thus lowering pH and causing oxidation of nitrite to nitrate. Figure 1 shows an overlay of chromatograms showing 2 mg/L nitrite, carbonate, nitrate, and sulfate in 1.6% NaCl brine, the standard, and the blank.

Figure 2: A typical breakthrough curve for a sample preparation cartridge.
An alternative experimental approach to measure capacity is to determine the "breakthrough volume" of a stationary phase–sample combination. A solution of sample of known concentration is passed through the cartridge and the output of the cartridge is collected in fractions for analysis or monitored with a detector that can measure matrix components. Figure 2 shows a typical breakthrough curve. Initially, as the sample is passed through the cartridge, matrix components are retained by the resin until all sites are occupied. Then, the sample matrix components begin to be eluted (VB), resulting in an increase in the baseline until the original concentration of sample is being eluted continuously (VM). By knowing the volume of sample passed through the cartridge or the flow rate and the initial sample–matrix concentration, one can determine the loading capacity of the cartridge resin and thus make sure that one doesn't inject a sample concentration that will exceed this value (maximum sampling volume or calculated mass). Similarly to the calculation approximation, one should back off on the matrix mass by about 20% to take into account matrix variability and to obtain the cleanest sample.
Off-Line and In-Line Matrix Elimination
The most common need for matrix removal arises from the presence of sample matrix components that interfere with the elution of the targets. Such interference can mean that matrix components are co-eluted with the target or otherwise change the retention, elution, or recovery of the target. Off-line matrix removal sample preparation has been applied to a wide variety of sample types (5–21).

Figure 3: Chromatograms of a dried distillers grains with solubles (DDGS) sample showing improvements in peak shapes and recovery of inositol phosphates. Chromatograms: (a) without treatment, (b) with OnGuard II RP only, and (c) with OnGuard II RP and Ag/H. Columns: Dionex CarboPac PA100 Guard and Analytical (250 mm à 4 mm); eluent: 25â500 mM HCl; flow rate: 1 mL/min; temperature: 30 °C; postcolumn derivatization; ferric nitrate in perchloric acid; detection: UV absorbance at 290 nm. Peaks: 1â4 = InsP2, 5â13 = InsP3, 14â20 = InsP4, 21â24 = InsP5, 25 = InsP6.
Figure 3 demonstrates a recent study on the determination of inositol phosphates in dried distillers grains with solubles (DDGS). Because the eluent is HCl, chloride needed to be removed from the sample so that the early elutions could occur only from the eluent, and not be affected by the chloride in the sample matrix. The off-line, two-layer silver–hydronium cartridge (OnGuard II Ag/H, Thermo Fisher Scientific) contains silver-form resin to remove chloride and also has a layer of hydronium-form cation-exchange resin at the outlet to trap any breakthrough Ag+ ion that might redissolve. The sample also contains hydrophobic species that are co-eluted with the targets. A reversed-phase resin (OnGuard II RP, Thermo Scientific) removed those species (22). The cartridges were used in series with the reversed-phase cartridge followed by the two-layer silver–hydronium cartridge. Recovery of phytate was monitored at every step in the method development, and the overall loss on recovery was 4.5% using this order of cartridges. Interestingly, the loss on recovery using the reverse order of cartridges was 22%.
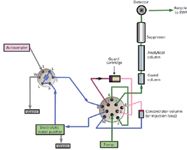
Figure 4: Example analytical setup for use of in-line sample preparation followed by preconcentration of the target analytes. The water for the loading pump should be cleaned using an electrolytic water purifier for minimal background.
An in-line version of sample preparation chemistries was introduced in the last couple of years, and its acceptance is growing (23). Use of in-line sample preparation chemistries has the advantage of requiring much less sample, as only the injection volume is treated, and can therefore be used repeatedly until the capacity is depleted. A concentrator column is used to "recollect" the targets for injection onto the analytical column. This arrangement minimizes operator time and saves money.
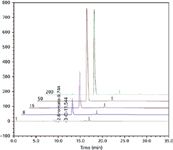
Figure 5: Comparison of lifetime chromatograms for 10 ppb bromate in 300 ppm chloride using sample preparation. Columns: IonPac AG24/AS24 (250 mm à 2 mm); eluent: 15â50 mM KOH gradient; flow rate: 0.3 mL/min; suppressor: ASRS 300 (2 mm); concentrator: TAC-ULP1; injection volume: 500 µL; sample preparation: InGuard Ag/InGuard H; sample loading: deionized water, 0.5 mL/min, 6 min.
Figure 4 shows a flow diagram for one configuration (of many) for use of in-line sample preparation. It is important to note that for low level determinations of common ions, the loading pump is usually supplied with electrolytically purified water (Electrolytic Water Purifier, Thermo Scientific or CIRA, Trovion Pte Ltd. Singapore) to minimize ions in the blanks from trace contamination in the loading water. Figure 5 shows the results of 200 injections of 10 ppb bromate in a matrix of 300 ppm chloride using in-line sample preparation. The relative standard deviation for retention time on 200 injections was 0.062%, the bromate peak area was 0.309%, and the amount (parts per billion), was 0.309%.

Table II: Retention time of polyphosphates in shrimp at the first and 300th injection, with and without using a sample preparation cartridge
A general application of in-line matrix removal in the food industry is the use of a hydrophilic styrene–divinylbenzene cartridge (InGuard HRP, Thermo Scientific) for the in-line removal of hydrophobic matrix components. Hydrophobic matrix components are usually unidentified but can be retained on reversed-phase stationary phases. One example is the determination of polyphosphates in shrimp (24). The goal was to prolong column life as evidenced by stable retention times over hundreds of injections. Table II shows the retention time data for the target polyphosphate species found in shrimp, comparing the first and 300th injection, with and without the use of in-line matrix removal.
Concentrators
Use of concentrators can lower detection limits by several orders of magnitude depending on the sample volume. Critical parameters include sample volume, capacity of the concentrator stationary phase, and composition of the sample matrix. Concentrators are used when the concentration of the target in the sample is too low for good quantification or when the sample band entering the analytical column is diffused, as in the case of some applications of in-line sample preparation. Some of the most common (and successful) uses of concentrators are in the determination of ultratrace targets in ultrapure water. In this case, the matrix to be eliminated is the water. Sometimes guard columns can be used as concentrators because the selectivity matches the analytical column. However, specifically designed concentrator columns are available with important features including ultralow back pressure for compatibility with an autosampler. Some concentrators have ultralow background of some analytes, such as sulfate (25). So once again, choices save time and money.
Summary
In this short review we have tried to cover the most commonly used sample preparation techniques for ion chromatography. The reference list provides many details that were outside the scope of this article. We began with the concept of choosing the best analytical column for the job; then, by understanding the chemistry issues associated with the sample, chose the best off-line or in-line matrix elimination solution.
References
(1) R. Slingsby and R. Kiser, Trends Anal. Chem. 20, 288–295 (2001).
(2) A. Seubert, W. Frenzel, H. Schafer, G. Bogenschutz, and J. Schaffer, Sample Preparation Techniques for Ion Chromatography, Metrohm Monograph, www.metrohm.com
(3) L. Bhattacharyya and J.S. Rohrer, Eds., Applications of Ion Chromatography in the Analysis of Pharmaceutical and Biological Products (John Wiley & Sons, February 2012), p. 207.
(4) R. Lin, K. Srinivasan, and C. Pohl, LCGC Europe 24(6), 322–332 (2011).
(5) Higher Resolution Separation of Organic Acids and Common Inorganic Anions in Wine, Application Note 273 (Thermo Scientific, Sunnyvale, California).
(6) Determination of Melamine in Milk by Ion Chromatography with UV Detection, Application Note 231 (Thermo Scientific, Sunnyvale, California).
(7) T. Phesatacha, S. Tukkeeree, and J. Rohrer, Determination of Phytic Acid in Soybeans and Black Sesame Seeds, Application Note 295 (Thermo Scientific, Sunnyvale, California).
(8) Y.S Ding and S.F. Mou, Chinese J. Chrom. (Zhongguo hua xue hui) 20(3), 262–264 (2002).
(9) Z.-X Guo et al., J. Anal. Atomic Spec. 18(11), 1396–1399 (2003).
(10) M.-L Jao et al., J. Food Drug Anal. 4(4) 349–358 (1996).
(11) S.D. Kumar, B. Maiti, and P.K. Mathur, Talanta 53(4), 701–705 (2001).
(12) X. Liu et al., Lizi Jiaohuan Yu Xifu/Ion Exch. Adsorption 23(6), 559–563 (2007).
(13) Y. Liu and S. Mou. Talanta 60(6), 1205–1213 (2003).
(14) Y. Liu, S. Mou, and D. Chen, J. Chrom. A 1039(1-2) 89–95 (2004).
(15) Y. Liu, S. Mou, and S. Heberling. J. Chrom. A 956(1–2), 85–91 (2002).
(16) B. Savary et al. Rev. Sci. Eau 13(2), 139–154 (2000).
(17) W. Shotyk, J. Chrom. 640(1-2), 309–316 (1993).
(18) W. Shotyk, I. Immenhauser-Potthast, and H.A. Vogel, J. Chrom. A 706(1-2), 209–213 (1995).
(19) J.E. Van Nuwenborg, D. Stöckl, and L.M. Thienpont, J. Chrom. A 770(1–2), 137–141 (1997).
(20) F. Wang et al., Aust. J. Chem. 57(10), 1005–1010 (2004).
(21) F.F. Zhang et al., Chinese J. Chrom./ Zhongguo hua xue hui 20(5), 452–455 (2002).
(22) Application note, Thermo Scientific 2013, in press.
(23) W. He et al., Chinese J. Chrom. (Se Pu) 30(4), 340–344 (2012).
(24) C. Chantarasukon, S. Tukkeeree, and J. Rohrer, Determination of Mono-, Di- and Triphosphates and Citrate in Shrimp by Ion Chromatography, Application Note 1007, (Thermo Scientific, Sunnyvale, California).
(25) Concentrator and Trap Columns, Thermo Fisher Dionex LPN 0913-16.
Rosanne Slingsby is a Senior Principal Scientist in the Research and Development Department at Thermo Fisher Scientific in Sunnyvale, California. She has worked in R&D for Dionex Corporation, now part of Thermo Fisher, for 31 years, developing a wide variety of products including the OnGuard and InGuard sample preparation cartridges. She is a co-author on two U.S. EPA methods for the determination of perchlorate and haloacetic acids by IC-MS and IC-MS-MS. Direct correspondence to: Rosanne.Slingsby@thermofisher.com

Rosanne Slingsby
Kassandra Oates received a B.S. in Biochemistry and Molecular Biology from the University of California in Santa Cruz, and has over 10 years of experience as an organic and analytical chemist. Prior to working at Thermo Fisher Scientific, Kassandra held positions at Elan Pharmaceuticals, Pickering Laboratories, Theravance Pharmaceuticals, and the Drug Enforcement Administration. Through these experiences she gained considerable experience using a variety of analytical instrumentations, including HPLC, NMR, GC, CE, IR, MS, and other related techniques. As an Applications Chemist at Thermo Fisher Scientific, Kassandra specializes in producing high quality applications documents to IC customers.

Kassandra Oates
Dong-bum Lee is an assistant technical support manager of the LC–IC–SP team in the Chromatography & Mass Spectrometry Division of Thermo Fisher Scientific. He obtained a Master's degree at Yong-in University in 2000 in Air Pollution. Dongbum worked for four years at the Korean Association of Industrial Health and joined Dionex Korea in 2004 as a chemist.

Dong-bum Lee

Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.










