Overview of Recent Development of Needle-Trap Devices for Analysis of Volatile Compounds
Needle-trap devices (NTDs) are another sorbent-based tool in the haystack of methods used in analytical extractions. Syringe needles, similar to those used for gas chromatography (GC) injection, can be partially filled with suitable sorbents and are used for extracting and collecting volatile organics, followed by injection into a GC instrument via thermal desorption. Although NTDs share many similarities and advantages of solid-phase microextraction (SPME), the larger sorbent bed provides robustness and offers potentially exhaustive extractions. This month, we take a look at the principles and applications of NTDs, and recent developments in their use.
In the early 1990s, Janusz Pawliszyn and his research group at the University of Waterloo (Canada) introduced solid-phase microextraction (SPME), and a new era in sample preparation for gas chromatography (GC) began. With SPME, analytes could be sampled by adsorption onto a stationary phase via direct immersion into a liquid sample or via headspace sampling of volatiles. Sample collection, extraction, clean-up, and concentration are combined into a single operation prior to injection into a chromatograph. Although SPME has seen applications with liquid chromatography (LC), the technique really made its mark as a sample preparation device for GC. We’ve somewhat recently provided an update to developments in SPME (1).
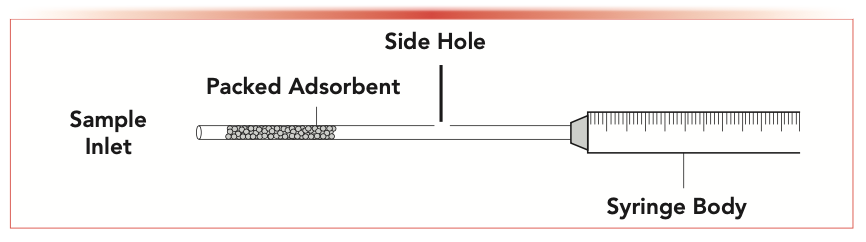
Perhaps most impressive about the development of SPME is its versatility, evidenced by new embodiments of the technique and spurring other sorptive-based extraction methods, such as thin-film microextraction (TFME), coated-blade spray (CBS) for mass spectrometry (MS), or stir-bar sorptive extraction (SBSE). Another of these unique geometries for SPME is the concept of needle-trap devices (NTDs) introduced by Koziel, Odziemkowski, and Pawliszyn in 2001 (2). Although different geometries exist, NTDs typically use syringe needles, generally the 23-gauge needles common for GC injection, packed with solid sorbent particles. NTDs are not to be confused with other packed-needle or syringe-tip approaches, like disposable pipet tips (DPX) or microextraction by packed sorbent (MEPS), which bear greater resemblance to conventional solid-phase extraction (SPE). Although, in theory, an NTD could be used with liquid samples, in practice the flow resistance limits these applications. An NTD configuration is displayed in Figure 1.
FIGURE 1: Schematic diagram of a needle-trap device (NTD) featuring an adsorbent packed inside a syringe needle, with an (optional) side hole in the syringe to facilitate air flow during sampling and carrier gas flow during desorption. Modified from reference (3) with the Creative Commons license.
Extraction with NTDs
With NTDs, given the greater amount of sorbent compared with conventional SPME approaches, diffusion of analytes into the needle and to the sorbent bed is not subject to equilibrium limitations. In fact, needle-trap extraction (NTE) is often considered exhaustive. The exhaustive nature of NTE removes the calibration considerations found with SPME and other non-exhaustive equilibrium extractions. The extraction mode typically occurs in conjunction with dynamic headspace sampling. This active sampling approach is possible because of the lack of diffusion limitations and serves to increase the speed of extraction. However, passive sampling approaches are also possible. Like SPME, NTE is a single-step procedure and solvent free (discounting the sorbent phase, which acts as the extracting solvent). Following extraction, the NTD can be stored for extended periods with little loss of analyte, then taken to the gas chromatograph, where, upon insertion into the injection port, analytes are thermally desorbed from the sorbent bed and move to the GC column. The needles packed as NTDs often have a side hole that provides three advantages: providing a portal for introducing the sorbent during the packing process; allowing for flow during dynamic headspace sampling; and, most importantly, facilitating flow of carrier gas in the GC inlet to stimulate quantitative transfer of desorbed analyte to the GC column. Some needle configurations feature a conical or extended tip to further direct analyte to the column.
Perhaps the biggest limitation of NTE is breakthrough volume: Once the sorbent bed becomes saturated with analyte, additional analyte is not collected in the needle trap. Breakthrough volume will primarily be a function of sorbent amount and surface area, and a plot of amount extracted versus analyte concentration in the sample is linear until breakthrough occurs. This allows quantitative extraction. Compared with standard SPME, NTE addresses concerns with fragility and adsorption capacity of the SPME fiber. Because there is more sorbent in an NTD, partition coefficient limitations are addressed, allowing extraction of analytes with smaller log P values. The time for an NTE is shorter than both SPME and traditional sorbent-based sampling tubes. All of the attributes of NTDs render them useful for on-site sampling or in- field analysis; for example, with occupational exposure studies. These features, combined with its solvent-free nature, give NTE serious green chemistry attributes. A recent review (4) provides a thorough discussion of the principles and practice of NTDs. NTDs have evolved to where they are now offered commercially by PAS Technology, Shinwa, PerkinElmer, and CTC Analytics.
Recent Developments with NTDs
Perusal of the NTD literature since 2020 reveals interesting trends. Particularly interesting are the extraction of aerosol droplets and particles, use of metal organic frameworks (MOFs) as sorbent phases, and breath analysis, including for disease diagnosis.
Extraction of Droplet-Bound and Gaseous Sample Components
Typically, the capture and analysis of droplet-bound and gaseous sample components, such as in breath analysis, are performed separately. Zeinali and Pawliszyn (5–8) have reported on combining a filter in front of the sorbent bed for a unified approach to this analytical challenge. The filter (for example, divinylbenzene) collects particulate matter and aerosol droplets, while Carboxen or other sorbent phases trap the volatile, gaseous compounds. They studied the stability of the droplets and gases in the air- sampling bags and obtained detection limits down to 0.05 mg/mL for their analysis. The method was applied with great success to pesticide aerosols, air from (burning) scented candles, and in the breath of volunteers following exposure to air freshener spray, fragrance mists, cannabis smoking, and incense.
Metal Organic Frameworks (MOFs) as Sorptive Media in NTDs
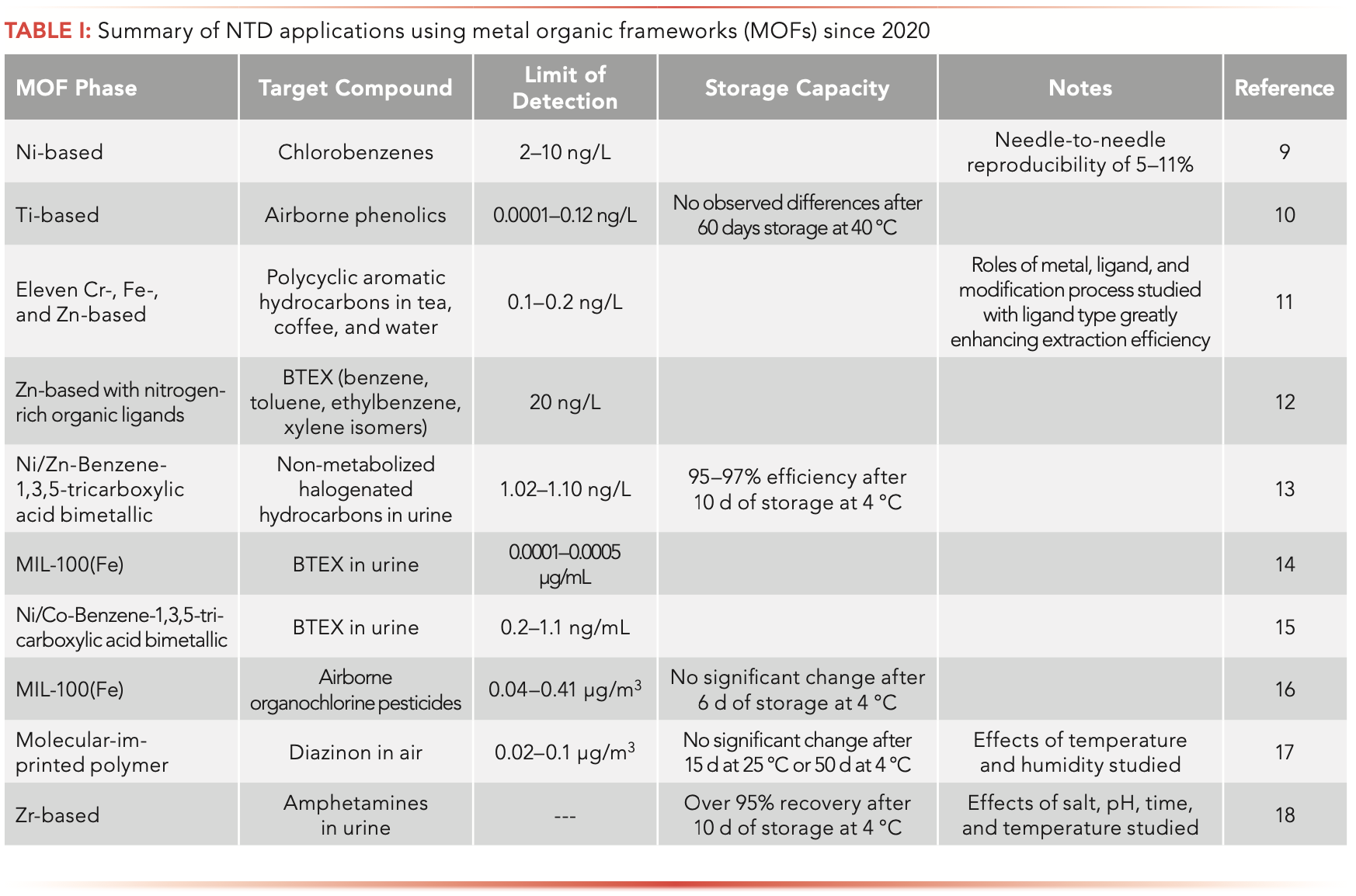
Development of newer phases to comprise the sorbent bed in NTDs is of ongoing interest. One type of media seeing wide development are MOFs. Table I summarizes selected recent applications of this technology. As a general rule, this approach provides sub-ng/mL limits of detection and exhibit minimal loss of analyte when the NTDs are stored at sub-ambient temperatures.
Other Sorptive Media
In addition to MOFs, other sorptive phases have been recently presented for NTD applications. A reduced graphine oxidemelamine formaldehyde phase created a superhydrophobic sorbent (19) with a reported 0.2 μg/L detection limit for chlorobenzenes. The NTD was reused over 200 times. An NTD with a Schiff base network-1/single-walled carbon nanotube (SNW-1/SWCNT) was created for phenolics analysis with limits of detection down to 0.002 ng m/L (20).
Disease Diagnosis
NTE for bovine respiratory disease diagnosis via cattle breath analysis was developed, though not enough evidence was obtained to create a useful profile of volatile organic compounds (VOC) (21). VOC analysis via NTE of fecal and tissue samples from cattle and goats identified a bacterial culture (22). With optimization, VOC emissions could be monitored for diagnosis of paratuberculosis in livestock.
Conclusion
As a unique embodiment of SPME, NTE presents a versatile, exhaustive extraction device for the sample preparation of volatile analytes prior to GC analysis. Continued development of needle configurations and interfacing with a GC inlet and types of sorbent media, including filter-sorbent combinations, will advance NTE into the everyday realm for occupational exposure, disease diagnostics, aerosol-bound analytes, and breath analysis.
References
(1) Raynie, D.E., Recent Advances in Solid-Phase Microextraction, Part I: New Tricks for an Old Dog. LCGC North Am. 2018, 36(3), 166–169.
(2) Koziel, J.A.; Odziemkowski, M.; Pawliszyn, J. Sampling and Analysis of Airborne Particulate Matter and Aerosols Using In-Needle Trap and SPME Fiber Devices. J. Anal. Chem. 2001, 73(1), 47–54. DOI: 10.1021/ac000835s
(3) Cheng, W.-H.; Lai, C.-H.; Tzeng, W.-J.; Her, C.; Hsu, Y.-H. Gaseous Products of Incense Coil Combustion Extracted by Passive Solid Phase Microextraction Samplers. Atmosphere 2015, 6(6), 822–833. DOI: 10.3390/atmos6060822
(4) Zeinali, S.; Khalilzadeh, M.; Pawliszyn, J.; The Evolution of Needle-Trap Devices with Focus on Aerosol Investigations. TrAC Trends Anal. Chem. 2022, 153, 116643. DOI: 10.1016/j.trac.2022.116643
(5) Zeinali, S.; Pawliszyn, J. Needle-Trap Device Containing a Filter: A Novel Device for Aerosol Studies. J. Anal. Chem. 2021, 93(43), 14401–14408. DOI: 10.1021/acs.analchem.1c01964.
(6) Zeinali, S.; Pawliszyn, J. Determination of Droplet-Bound and Free Gas-Phase Fragrances Using a Filter-Incorporated Needle-Trap Device and Solid-Phase Microextraction Technologies. J. Agric. Food Chem. 2021, 69, 13657– 13667 (2021). DOI: 10.1021/acs.jafc.1c06006
(7) Zeinali, S.; Ghosh, C.; Pawliszyn, J. Simultaneous Determination of Exhaled Breath Vapor and Exhaled Breath Aerosol Using Filter-Incorporated Needle-Trap Devices: A Comparison of Gas-Phase and Droplet-Bound Components. Anal. Chim. Acta 2022, 1203, 339671. DOI: 10.1016/j.aca.2022.339671
(8) Zeinali S.; Pawliszyn, J. Effect of Household Air Pollutants on the Composition of Exhaled Breath Characterized by Solid-Phase Microextraction and Needle-Trap Devices. Anal. Bioanal. Chem. 2022, 414(18), 5573–5583. DOI: 10.1007/s00216-022-03997-6
(9) Javanmardi, H.; Abbasi, A.; Bagheri. H. The Geometrical Characteristics of Nickel-Based Metal Organic Framework on its Entrapment Capability. J. Chromatogr. A 2020, 1610, 460551. DOI: 10.1016/j.chroma.2019.460551
(10) Firoozichahak, A.; Bahrami, A.; Ghordani Shahna, F.; Aizadeh, S.; Nematollahi, D.; Farhadian, M. Development of a Needle Trap Device Packed with Titanium-Based Metal-Organic Framework Sorbent for Extraction of Phenolic Derivatives in Air. J. Sep. Sci. 2020, 43, 1011–1018. DOI: 10.1002/jssc.201900938
(11) Javanmardi, H.; Abbasi, A.; Bagheri, H. Roles of Metal, Ligand, and Post Synthetic Modification on Metal Organic Frameworks to Extend their Hydrophobicity and Applicability Toward Ultra-Trace Determination of Priority Organic Pollutants. Anal. Chim. Acta 2020, 1125, 231–246. DOI: 10.1016/j.aca.2020.05.064
(12) Noorpoor, Z. The Needle Trap Extraction Capability of a Zinc-Based Metal Organic Framework with a Nitrogen Rich Ligand. J. Coord. Chem. 2021, 74(13), 2213–2226. DOI: 10.1080/00958972.2021.1962524
(13) Rahimpoor, R.; Firoozichahak, A.; Nematollahi, D.; Alizadeh, S.; Alizadeh, P.M.; Langar, A.A.A. Determination of Halogenated Hydrocarbons in Urine Samples Using a Needle Trap Device Packed with Ni/Zn–BTC bi-MMOF via the Dynamic Headspace Method. RSC Advances 2021, 11, 21537–21547. DOI: 10.1039/D1RA03227E
(14) Saedi, N.; Bahrami, A.; Ghorbani Shahna, F.; Mohraz, M.H.; Faradian, M.; Alizadeh, S. A Needle Trap Device Packed with MIL-100(Fe) Metal Organic Frameworks for Efficient Headspace Sampling and Analysis of Urinary BTEXs. Biomed. Chromatogr. 2020, 34, e4800. DOI: 10.1002/bmc.4800
(15) Rahimpoor, R.; Firoozichahak, A.; Nematollahi, D.; Alizadeh, S.; Alizadeh, P.M.; Langari, A.A.A. Bio-Monitoring of Non-Metabolized BTEX Compounds in Urine by Dynamic Headspace-Needle Trap Device Packed with 3D Ni/Co- BTC Bimetallic Metal-Organic Framework as an Efficient Absorbent. Microchem. J. 2021, 166, 106229. DOI: 10.1016/j.microc.2021.106229
(16) S. Soury, A. Firoozichahak, D. Nematollahi, S. Alizadeh, H. Kakaei, and A. Abbasi. Needle-Trap Device Packed with the MIL-100(Fe) Metal–Organic Framework for the Extraction of the Airborne Organochlorine Pesticides. Microchem. J. 2021, 171, 106866. DOI: 10.1016/j.microc.2021.106866
(17) Rahimpoor, R.; Firoozichahak, A.; Alizadeh, S.; Soleymani-Ghoozhdi, D.; Mehregan, F. Application of a Needle Trap Device Packed with a MIP@MOF Nano-Composite for Efficient Sampling and Determination of Airborne Diazinon Pesticide. RSC Advances 2022, 12, 16267–16276. DOI: 10.1039/D2RA01614A
(18) Rahimpoor, R.; Firoozichahak, A.; Alizadeh, S.; Nematollahi, D.; Urinary Bio-Monitoring of Amphetamine Derivatives by Needle Trap Device Packed with the Zirconium-Based Metal–Organic Framework. Sci. Reports 2022, 12, 13702. DOI: 10.1038/s41598-022-17861-1
(19) Zare, F.D.; Allahdadlalouni, M.; Baktash, M.Y.; Bagheri, H. Reduced Graphene Oxide–Melamine Formaldehyde as a Highly Efficient Platform for Needle Trap Microextraction of Volatile Organic Compounds. Microchem. J. 2020, 157, 104932. DOI: 10.1016/j.microc.2020.104932
(20) Safarpour Khotbesara, N.; Bahrami, A.; Habibi Mohraz, M.; Afkhami, A.; Farhadian, M. Development of a Needle Trap Device Packed with the Schiff Base Network-1/Single-Walled Carbon Nanotube for Sampling Phenolic Compounds in Air. Microchem. J. 2022, 172, 106984. DOI: 10.1016/j.microc.2021.106984
(21) Haddadi, S.; Koziel, J.A.; Engelken, T.J. Analytical Approaches for Detection of Breath VOC Biomarkers of Cattle Diseases- A Review. Anal. Chim. Acta 2022, 1206, 339565. DOI: 10.1016/j.aca.2022.339565
(22) Safarpour Khotbesara, N.; Bahrami, A.; Habibi Mohraz, M.; Afkhami, A.; Farhadian, M. Development of a Needle Trap Device Packed with the Schiff Base Network-1/Single-Walled Carbon Nanotube for Sampling Phenolic Compounds in Air. Microchem. J. 2022, 172, 106984. DOI: 10.1016/j.microc.2021.106984
ABOUT THE COLUMN EDITOR
Douglas E. Raynie
“Sample Prep Perspectives” editor Douglas E. Raynie is a Department Head and Associate Professor at South Dakota State University. His research interests include green chemistry, alternative solvents, sample preparation, high-resolution chromatography, and bioprocessing in supercritical fluids. He earned his PhD in 1990 at Brigham Young University under the direction of Milton L. Lee. Raynie is a member of LCGC’s editorial advisory board. Direct correspondence about this column via e-mail to LCGCedit@mjhlifesciences.com


Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.