Optimizing Splitless GC Injections
Splitless injections are sometimes necessary for trace analyses, where the analyst hopes to recover 100% of the analytes that are injected. Unfortunately, splitless injections can be challenging and using an imperfect method can lead to loss of analytes and poor peak shapes. The choice of inlet liner can have an impact on the data and one must consider the effects of geometry, packing, deactivation, and volume on introduction of analytes into the system. Other important inlet parameters to consider include inlet temperature, splitless hold time, and initial oven temperature.
Photo Credit: MF production/Shutterstock.com

Splitless injections are sometimes necessary for trace analyses, where the analyst hopes to recover 100% of the analytes that are injected. Unfortunately, splitless injections can be challenging and using an imperfect method can lead to loss of analytes and poor peak shapes. The choice of inlet liner can have an impact on the data and one must consider the effects of geometry, packing, deactivation, and volume on introduction of analytes into the system. Other important inlet parameters to consider include inlet temperature, splitless hold time, and initial oven temperature.
Split and splitless injections are two very common techniques used for the introduction of samples into a gas chromatograph. Split injections generally provide superior chromatography because of the rapid transfer of the sample through the inlet onto the column, leading to sharp peaks with little time for adverse interactions to occur within the inlet. The drawback of split injections is that the majority of the sample is vented and lost, making it sometimes necessary to use a splitless injection when performing trace analyses.
During a splitless injection, the split vent is closed, with the total inlet flow essentially being equal to the column flow. Because capillary column flows are typically slow in relation to the total inlet volume, it can be difficult to transfer the sample to the column in a tight band, leading to broadened or poor peak shapes. The longer residence time of the sample in the hot inlet can also lead to adverse interactions, such as adsorption and chemical reactivity. Optimizing splitless injections requires careful selection of an inlet liner, as well as using an appropriate inlet temperature, splitless hold time, initial oven temperature, and solvent.
Liner Selection
Geometry and Packing: Glass inlet liners serve as a chamber in which a liquid sample is vapourized and transferred to the column. The shape of a liner, as well as the presence or absence of packing material, can affect its heat capacity and flow dynamics, ultimately affecting both the responses of compounds, as well as the injection to injection repeatability (1).
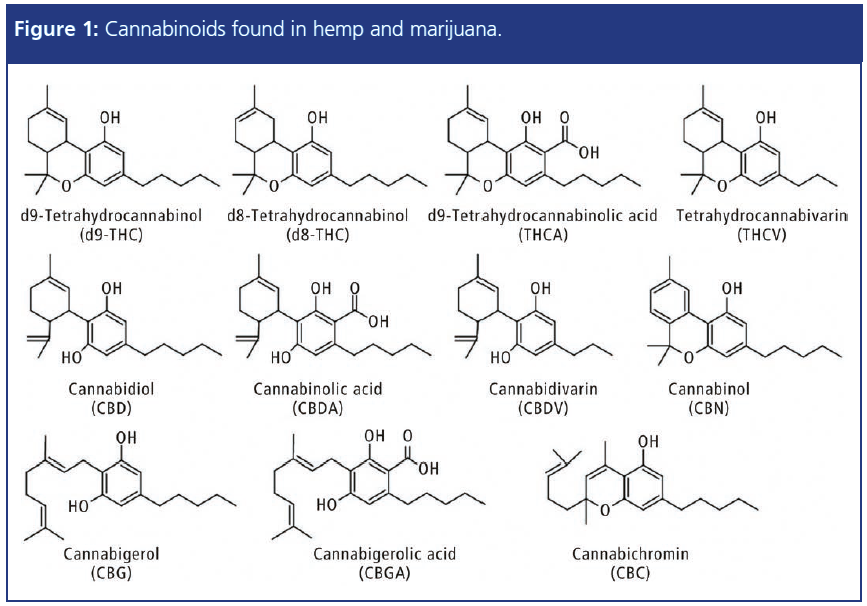
Figure 1 shows a comparison of hydrocarbon peak area responses for liners commonly used with splitless injections. The single taper liner containing glass wool, as well as the double taper cyclo liner, which features a corkscrew obstruction, demonstrated the best overall responses across all hydrocarbons ranging from C8 up to C40. Both the wool and the corkscrew provide additional surface area, which increases the heat capacity of the liner and allows for better vapourization of analytes with high boiling points. Wool and the corkscrew can also help to increase reproducibility by enhancing mixing with the carrier gas. In addition to these benefits, wool can protect the column by trapping relatively nonvolatile matrix material as well as particles and pieces of septa.
In contrast, the empty straight liner and single taper liner show increasingly poor vapourization potential as the analyte boiling points increase. This phenomenon is known as molecular weight discrimination. If analyzing analytes with relatively low boiling points, using wool or some type of obstruction may not be necessary for complete vapourization; however, column maintenance intervals may be more frequent, with shorter overall lifetime, because of the lack of protection from nonvolatile matrix components.
A bottom taper is recommended when performing splitless injections because it helps to direct the sample to the column, while also minimizing contact with the seal. The inlet seal is often cooler and contact with it can condense high molecular weight analytes, leading to losses.
Inertness: Liners are usually constructed from borosilicate glass, which contains active sites such as silanols, as well as metallic impurities. These active sites may interact with sensitive analytes, leading to adsorptive loss, as well as chemical reactivity. Adverse interactions are far more likely to occur with splitless injections because inlet flows are slow and analyte residence times are relatively long. A surface treatment, or deactivation, is needed to cover active sites and produce an inert surface with which analyte integrity will remain.
Deactivation typically involves the silanization of the liner surface and can be performed with either liquid or gas phase reagents. Dimethyldichlorosilane (DMDCS), a commonly used glassware deactivation reagent, is used as a liquid deactivant and the procedure is simple enough that most laboratories can perform it in-house. Many instrument and consumables manufacturers use a chemical vapour deposition of proprietary deactivation reagents instead because superior inertness is often achieved. Deactivation quality can vary depending on the process used, and will affect responses of active analytes, so it’s important to seek out a deactivation that works for your particular analytes.
The benefits of using wool were discussed earlier; however, the high surface area of wool can also make it difficult to thoroughly deactivate, potentially exposing analytes to active sites. Both borosilicate and quartz wool are used in liners, with quartz offering superior inertness because it contains less impurities. There are liners pre-packed with quartz wool that has been deactivated, allowing for the use of wool, even with active analytes. Nonetheless, in some cases it may be necessary to select a liner without wool.
Using a liner with a bottom taper is beneficial for splitless injections because it helps to lessen or prevent contact with the bottom metal seal of the inlet, where analytes can also react.
Volume: Upon injection of a liquid sample into a hot inlet liner, solvents will greatly expand as they are transferred to a gas. If the solvent expands beyond the confines of the liner, gas lines can potentially become contaminated and reproducibility may suffer. This phenomenon is referred to as backflash. The expansion volume will depend on the solvent, inlet temperature, and inlet pressure. Using a liner with a larger inner diameter (3–5 mm) is usually preferred for splitless injections because it allows the most expansion volume for the solvent.
If using a solvent with a low expansion coefficient, it may be possible to use a liner with a narrower inner diameter. The advantage of this is faster transfer of analytes to the column, which helps to prevent band broadening and allows less time for adverse interactions to occur within the liner.
Inlet and Oven Parameters
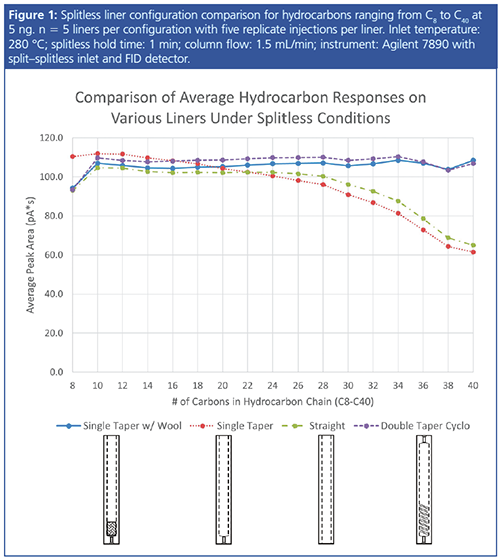
Inlet Temperature: Inlet temperature can have a profound impact on analyte response because higher temperatures provide increased thermal energy for transferring semivolatile compounds to a gaseous state. Unfortunately, high inlet temperatures can also cause increased degradation of thermolabile compounds. It is often necessary to find an inlet temperature that provides a good balance of response for high boilers as well as minimal degradation of sensitive compounds.
Figure 2(a) shows the response of benzo[ghi]perylene, a polycyclic aromatic hydrocarbon (PAH) with a boiling point of 550 ºC, at various inlet temperatures ranging from 200 ºC to 300 ºC. Note that the gains in peak area become increasingly less significant as the temperature continues to increase beyond 250 ºC. On the other hand, endrin, a thermolabile chlorinated pesticide, shows greater chemical reactivity at higher inlet temperatures, leading to increased formation of its degradation products, endrin aldehyde and endrin ketone (Figure 2[b]).
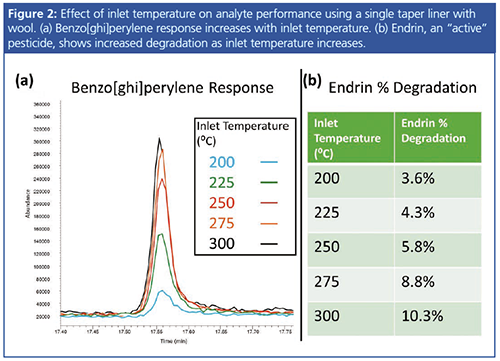
Splitless Hold Time: During the splitless hold time, all inlet flow is directed to the column, as analytes evaporate within the inlet and transfer to the column. This will result in a large amount of solvent being transferred to the column relative to the analytes. If too much solvent is loaded onto the column, it can potentially interfere with early-eluting analytes or lead to an elevated baseline; therefore, at a predetermined time, the split vent must be opened, allowing excess solvent to exit the inlet. On the other hand, if the splitless hold time is too short, there may be insufficient vapourization and transfer of analytes.
Figure 3 demonstrates the effect of splitless hold time on analyte and solvent response. Allowing a 1.5 to 2 times sweep of the total inlet liner volume with carrier gas before opening the split vent should provide time for most analytes to achieve sufficient evaporation, assuming all other parameters are also optimized. A shorter hold time may be needed if the solvent peak interferes with the most volatile compounds.
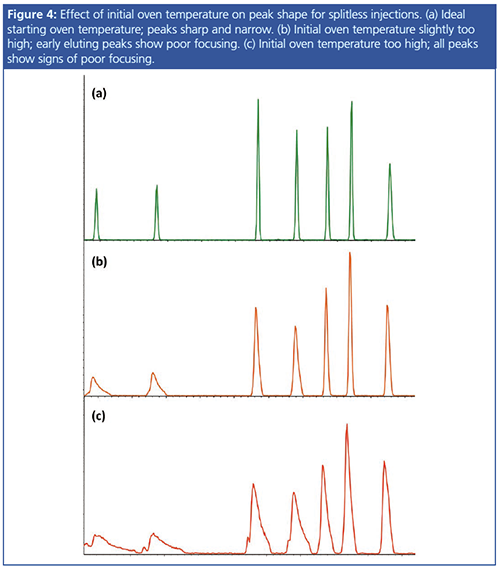
Initial Oven Temperature: The low flow rates inherent to splitless injections mean that complete transfer of the analytes to the column can be slow, leading to diffusion, or band broadening, of analytes. This ultimately leads to wider peaks, affecting quantitation and detection limits. To counteract this unwanted effect, it is necessary to condense the analytes at the head of the analytical column. This is achieved by using a low initial oven temperature.
Starting at an oven temperature below the boiling point of the solvent will condense the solvent at the head of the column, which will then trap analytes (“solvent effect”). This will yield a narrow starting band from which the chromatographic separation can begin. If the analytes of interest have a substantially higher boiling point than the solvent (>200 °C), an alternative method is to set the starting oven temperature below the boiling point of the earliest eluting analyte. This will ensure analytes condense at the head of the column in a tight band, even if the solvent does not. Figure 4 demonstrates the effect of initial oven temperature on peak shape.
Solvent Polarity: Matching the solvent polarity to the column polarity is important for good peak shape. The general chemistry rule of “like dissolves like” applies here, as injecting a polar solvent like methanol onto a nonpolar column such as a 1-type (dimethyl polysiloxane) will result in the solvent beading up rather than dissolving into the column’s phase. This can lead to split and deformed peak shapes with low reproducibility.
Final Considerations
Successful splitless analyses require careful optimization of a number of parameters. Keep in mind that many of these parameters are interrelated and the choice of one can affect others. For instance, using a liner with a higher heat capacity may lead to the ability to use a lower inlet temperature set point. Using a lower inlet temperature may require a longer splitless hold time and so on. Understanding the effect of adjusting each parameter is the ultimate goal for effective method development; there isn’t a “one-size-fits-all” method that will work for every analysis. The chemical properties of the analytes, as well as the solvent used, will impact the optimal conditions for a particular splitless method.
Reference
- L. Waclaski, American Laboratory48(6), 24–26 (2016).
Linx Waclaski is a chemist in Restek’s GC Applications group, with his primary focus being on the GC accessories product line. Prior to joining Restek in 2013, he worked in an environmental testing laboratory performing various semivolatile organics analyses using GC. Linx received a master’s degree in forensic science and a bachelor’s degree in biochemistry from Duquesne University (Pennsylvania, USA).

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.