Non-Discriminating Analytical Pyrolysis — A Novel Tool for Studying Environmental Samples
LCGC Europe
Conventional pyrolysis can cause defunctionalization of polar structural moieties carrying functional groups, which leads to biased results.
Analytical pyrolysis is a convenient and efficient technique for obtaining structural information on both natural and synthetic polymers through the release of characteristic fragments on rapid thermal degradation. However, commercial pyrolysis systems usually suffer from discrimination of high molecular weight compounds on transfer from the pyrolysis unit to the gas chromatograph. This limits the amount of information that can be gained from pyrolysis experiments. ...To eliminate this instrument-based limitation, the technique of non-discriminating pyrolysis based on capacitive discharge heating of a Silcosteel tube filled with the pyrolysed sample was introduced in 2001. This technique has since been applied for the characterization of many different types of samples, including soil, bacteria and airborne particulate matter with minimum or no sample preparation. ...The approach requires very small amounts of sample material and is fast and simple. A combination of the non-discriminating pyrolysis system with comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry (GC×GC/TOF-MS) creates a very powerful tool for the characterization of extremely complex analyte mixtures.
Pyrolysis has established itself as an important analytical technique to characterize high-molecular weight organic compounds including biopolymers, synthetic polymers (homo-and copolymers) and complex natural matrices (microorganisms, body fluids, plant tissues and raw materials). Intact macromolecular samples cannot be subjected to GC combined with mass spectroscopy (MS), atomic emission detection (AED), etc. To detail the structure of polymers, it is necessary to generate significant, vaporizable thermal fragments without secondary reactions.
Non-degradative spectroscopic methods, including nuclear magnetic resonance (NMR), fourier transform infrared (FTIR) and ultraviolet (UV), provide information on functional groups and steric arrangements between the subunits. A degradative method such as pyrolysis analyses building blocks in long sequences in polymeric chains that contain essential structural information, thus supplementing "gross-chemical-composition" related data.1 Pyrolysis profiles are regarded to be characteristic for a particular sample, either in the appearance of unique compounds, or in the relative distribution pattern of the pyrolysis products.2 Pyrolysis gas chromatography–mass spectrometry is a powerful tool for fingerprint characterization of complex organic materials, including natural organic polymers such as humic organic matter (HOM) and artificial polymers (e.g., plastics).3–5 Element-specific AED is sometimes used to supplement the MS data.
Conventional pyrolysis using commercially available devices might in some instances face significant limitations.6 High molecular weight fragments produced during pyrolysis of both natural and synthetic materials, often carrying very significant structural information, are frequently lost on transfer from the pyrolysis unit to the gas chromatograph. Conventional pyrolysis systems require some form of interface between the pyrolyzer and the gas chromatograph. Such interfaces often have large internal volumes. Less volatile pyrolysis products might dwell in the unswept areas of the interface. In addition, there are usually some cold spots in the interface. Quite frequently, the interfaces are in fact designed not to allow high-molecular weight compounds into the GC column, to prevent problems with system contamination. This may lead to loss of valuable information.
To overcome these problems, a new technique, called non-discriminating pyrolysis, was introduced in 2001.6,7 The technique makes it possible to analyse high molecular weight fragments produced during pyrolysis. By overcoming the limitations of conventional systems, non-discriminating pyrolysis makes it possible to tackle some analytical problems that could not be dealt with efficiently thus far. The ability of the technique to analyse high-molecular weight pyrolysis products is illustrated in Figure 1, which shows a comparison of polyethylene pyrograms obtained with a commercial system (top) and with the non-discriminating pyrolysis system (bottom). With the commercial system, the usual α,ω-alkadiene/n-alk-1-ene/n-alkane pattern extended only to about C-35. However, fragments as large as C-73 (M.W. 1020/1022/1024), which is practically the limit of gas chromatography, could be observed with the non-discriminating system. Thus, the range of pyrolysis products amenable to analysis with the non-discriminating system is limited by gas chromatography rather than by the pyrolysis system itself.
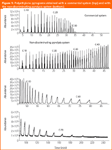
Figure 1: Polyethylene pyrograms obtained with a commercial system (top) and with the non-discriminating pyrolysis system (bottom).
Conventional pyrolysis can cause defunctionalization of polar structural moieties carrying functional groups, which leads to biased results. As an example, in the past, models of humic organic matter structure were made based on pyrolysis data. These proposals proved inconsistent with spectroscopic, as well as microanalysis data ("bulk" elemental C/O-ratios) because of defunctionalization of many compounds of interest (e.g., carboxylic acids). In addition, very polar pyrolysis products are often incompatible with GC columns. One possible solution to this problem is the application of a methodological variation of conventional pyrolysis, namely thermochemolysis. In this technique, a chemical reagent, such as tetramethylammonium hydroxide (TMAH) or trimethylsulphonium hydroxide (TMSH), is added to the sample. Upon heating to thermochemolysis temperature (usually lower than pyrolysis temperature), TMAH or TMSH hydrolyse large molecules, breaking them apart while protecting the functional groups through methylation, making them amenable to GC.8
Experimental
Experiments were performed using a GC–MS system (HP6890/HP5973) from Agilent Technologies, Mississauga, Ontaria, Canada. Data acquisition was performed in full-scan mode. Columns used included 32 m × 0.25 mm × 0.1 μm DB-1 HT (J&W), 30 m × 0.25 mm × 0.25 μm Solgel-Wax (SGE) and (30 + 10) m × 0.25 mm × 0.25 μm EZ-guard VF-5ms (Varian).
All columns except EZ-guard VF-5ms were equipped with 2 m fused-silica precolumns, connected with glass press-fit connectors (Chromatographic Specialties, Brockville, Ontario, Canada). Different temperature programmes were used, depending on the requirements of the particular analysis, but in general, the oven was kept at 40 °C for 2 or 5 min, and then the temperature was programmed to 360 °C, 320 °C or 280 °C, depending on the column, at rates varying from 8 to 20 °C/min.
The GC×GC/TOF-MS system included an Agilent 6890 gas chromatograph and Pegasus III GC/TOF-MS equipped with a single jet cryogenic modulator with a delay loop.9 The column used in the first dimension was VF-5MS, 30 m × 0.25 mm × 0.25 μm; the second dimension column was VF-25MS, 1.6 m × 0.1 mm × 0.1 μm.
Figure 2 presents a schematic diagram of the non-discriminating pyrolysis system.7,10 Pyrolysis was performed in an inert Silcosteel metal capillary (0.53 mm i.d.; Restek, Bellefonte, Pennsylvania, USA). This capillary was disposable and was used only once, unless sequential heating of the sample at progressively higher temperatures was required. The sample was kept in place inside the capillary by means of two fused-silica wool plugs (Quartz Fiber Filter, F&J, Specialty Products Inc., Florida, USA).

Figure 2: Schematic diagram of the non-discriminating pyrolysis system.7 1 = restrictor, 2 = union, 3 = silcosteel capillary, 4 = quartz fibre plugs, 5 = sample, 6 = ceramic shield, 7 = top of GC oven, 8 = GC injection port, 9 = GC column (precolumn)
A stainless steel restrictor (50 μm i.d.; Micro Group Inc.) supplying auxiliary carrier gas was connected to the upstream end of the capillary through a Graphpack connector (Gerstel Inc., Baltimore, Maryland, USA). The downstream end of the pyrolysis capillary was connected to a modified GC injector. The pyrolysis capillary was heated rapidly to the pyrolysis temperature by passing electric current from a custom-built capacitive discharge power supply connected to the capillary through fixed contacts.7 Following the heating pulse, the capillary was kept at a sustained temperature of 360 °C for 2 min to facilitate transfer of high molecular weight products to the gas chromatograph. This post-heating was accomplished by passing direct current from the power supply through the capillary.
In the analysis of fine airborne particulate matter (PM2.5 ), direct thermal desorption was applied. Urban airborne PM2.5 was collected on pre-fired quartz-fibre filters (47 mm i.d.) within the National Air Pollution Surveillance (NAPS) PM2.5 speciation air quality monitoring network. For the experiments, ~2 mg of the quartz filter used in collection of PM2.5 was placed inside the Silcosteel capillary. Heat was applied at different temperatures (between 120 °C and 550 °C) for thermal desorption by sustained electrical current provided by the power supply.
Non-discriminating thermochemolysis of all samples mentioned in this article was performed by injecting two 0.5 μL aliquots of 25% tetramethylammonium hydroxide (TMAH) solution in MeOH into the pyrolysis capillary containing the samples, one upstream and one downstream of the sample. The TMAH solutions were prepared fresh each day. Thermochemolysis was performed at 500 °C.
Results and Discussions
Application of non-discriminating pyrolysis in soil analysis: Soil analysis is essential in studying carbon dynamics and carbon stabilization processes (carbon transformation, carbon turnover rates, soil as sink or source of atmospheric carbon dioxide etc.). Soil organic matter dynamics affects soil fertility, soil aggregation, resistance to physical degradation/erosion, climate change and others. As an example, the purposeful formation of stabilized soil organic matter can counteract the greenhouse effect by soil acting as methane (and possibly other greenhouse gases) sink.
A number of analytical techniques including non-degradative (spectroscopic) and degradative ones (pyrolysis, chemical methods including hydrolysis or selective oxidation etc.) can be used for the structural characterization of soil organic matter (SOM).11–13
Most of the organic matter in soils is present in macromolecular structures that can only be investigated by GC–MS using degradative techniques. Nowadays, an urgent challenge in soil analysis is to detect the molecularly-uncharacterized soil fraction (the well-cited "the carbon we do not see"). As a rough estimate, about one half of all organic matter in soils still remains uncharacterized on the molecular level. The non-discriminating pyrolysis/thermochemolysis approach might help fill this gap. When using conventional pyrolyzers, conclusions regarding the original structure of the SOM in the samples must be drawn with caution because of the discrimination of high molecular weight compounds carrying high diagnostic value (e.g., long-chain hydrocarbons/alcohols/2-ketones, sterols, hopanes etc.), which may lead to misinterpretation of the results. The non-discriminating pyrolysis technique can potentially lead to less biased results.
The technique was used for structural characterization of SOM. Its usefulness in this type of application is illustrated in Figure 3. A peat sample was TMAH-thermochemolysed using the system described above (see Figure 2) and a SolGel-Wax column without any additional sample preparation. Plant-derived tocopherol was one of the analytes detected among the thermochemolysis products of peat. Other interesting high-molecular weight products detected in this sample included hopanes and hopanoids (hopanols, hopanoic acids) originating chiefly from methanotrophic bacteria.9 These molecules, well known for their high recalcitrance, cannot usually be unambiguously characterized with commercial pyrolysis systems because of discrimination during transfer from the pyrolyser to the gas chromatograph

Figure 3: Identification of plant-derived tocopherol among the products of TMAH-induced thermochemolysis (500 °C) of a peat sample using the selected ion m/z = 430 amu (full scan data acquisition).
It should be pointed out that the sample was subjected to exhaustive solvent extraction before pyrolysis/thermochemolysis. Thus, we can conclude that the hopane hydrocarbons, as well as the hopanoic acids — both originating from bacterial hopanepolyols — are covalently bound inside the macromolecular HOM network. Although quantification of the hopanes/hopanoids released on pyrolysis/thermochemolysis still needs to be detailed, this finding may foster the molecularly-based search of soil organic matter building blocks responsible for soil recalcitrance. The SolGel-Wax column is particularly suited for this type of analysis because of its high thermal stability (up to 300 °C in temperature-programmed mode).
Microbial community structure analysis by fatty acid profiling. TMAH-induced thermochemolysis can be a very useful tool in the determination of soil microbial biomass, which is a small, but very labile fraction of SOM. Non-discriminating TMAH-induced thermochemolysis enables the determination of fatty acid methyl ester (FAME) patterns along with plant or fungal sterols in soil samples with practically no sample preparation, while requiring only very small amounts of the sample. Lipid diagnostic fatty acid biomarkers were studied in soil samples that underwent different fertilization treatments. They provided important information on the dynamics of carbon pools, stabilization processes of soil organic matter, significance of fertilization treatments etc. On the basis of fatty acid patterns, mostly originating from membrane phospholipids, conclusions could be drawn on the soil microbial community structure. Figure 4 shows mirror images of the pyrograms of a soil sample treated with green manure and soil without any treatment, respectively (extracted ion m/z 74), obtained by thermochemolysis with 25% TMAH. The pyrograms clearly illustrate that the microbial population was affected by the organic substrate in the green manure fertilizer both quantitatively (higher FAME content in the fertilized sample as compared to the Fallow sample) and qualitatively (the presence of C18:1ω7 surrogates indicating bacterial input, while higher abundances of FAMEs beyond C18 indicating plant origin). The FAME surrogates beyond C18 (C30 and above) often cannot be detected unambiguously when using conventional pyrolysis systems, resulting in biased results.
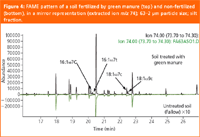
Figure 4: FAME pattern of a soil fertilized by green manure (top) and non-fertilized (bottom), in a mirror representation (extracted ion m/z 74); 63â2 μm particle size; silt fraction.
In addition to studying microbial communities in soil, the non-discriminating thermochemolysis using TMAH can also be used for studying bioremediation processes, stress phenomena and fermentation protocols. Chemical or thermal stress can modify the fatty acid patterns (see reference 14 and references cited therein) through cis/trans isomerization of monounsaturated fatty acids. A prerequisite to trace these processes is the availability of an analytical technique able to preserve the cis/trans ratio of these acids. It was conclusively demonstrated using Pseudomonas putida that the technique proposed meets this essential requirement.14 Figure 5 shows an example of a FAME pattern obtained using non-discriminating thermochemolysis for Gram-negative Pseudomonas putida. The patterns obtained by this method were compared with patterns obtained by classical preparation schemes (pressurized solvent extraction, alkaline saponification) and by using conventional pyrolysis units. No significant discrimination could be observed with the system proposed for saturated, monounsaturated and cyclopropane fatty acids (FA) when using thermochemolysis temperatures up to 550 °C. The cis/trans ratio of monounsaturated FA was preserved with the non-discriminating system, which was not the situation when conventional pyrolysis units were used. Hydroxy-FA located in polysaccharides of Gram-negative bacteria, therefore eluding solvent extraction, could be almost quantitatively liberated by TMAH using non-discriminating pyrolysis, whereas the recoveries were lower with conventional approaches.

Figure 5: Bacterial fatty acids methylated by boron trifluoride-methanol (top) and by TMAH-induced thermochemolysis at 500 °C (bottom). Extracted ion m/z 55; intensity of the ion chromatograms normalized to the internal standard (reprinted from reference 14 with permission from Elsevier).
Characterization of PM2.5 : Identification of novel marker compounds from major emission sources (anthropogenic, biogenic, synthetic, geogenic) and from secondary photochemical processes is essential for improving the detail of fine airborne particulate matter (PM2.5 ) organic chemical composition characterization. One of the methods commonly used in the analysis of PM is thermal desorption followed by GC–MS to identify and measure the molecular markers. The non-discriminating pyrolysis system can be used for both thermal desorption and pyrolysis, therefore its usefulness for the characterization of PM2.5 was evaluated.
Thermal desorption of PM2.5 samples collected on quartz filters was performed at temperatures of 120, 250, 450 and 550 °C. The volatile compounds evolving from the samples were analysed by GC–MS and comprehensive GC×GC/TOF-MS. This approach is faster, more environmentally friendly and requires much less material than solvent extraction methods. GC×GC is capable of separating extremely complex mixtures of compounds with a concurrent increase in sensitivity, therefore it is an ideal tool for the analysis of the organic fraction of airborne particulate matter. Families of compounds can often be easily identified in the GC×GC chromatograms because of their structured nature. The TOF-MS detection method allows the deconvolution of the mass spectra of co-eluting compounds, which further improves the identification of the analytes.
PM2.5 contains many different organic compounds. Figure 6 shows the extracted ion trace for ion m/z 91, characteristic of alkyl-substituted benzenes, using conventional GC–MS and a desorption temperature of 450 °C. Only a small fraction of the semi-volatile organic compounds (SVOC) present in urban air particulate matter samples could be identified using this technique because of the insufficient chromatographic resolution for samples as complex as PM volatiles and semi-volatiles, which results in numerous coelutions. Consequently, identification of organic compounds in urban air particulate matter using thermal desorption combined with GC–MS is often biased. The use of GC×GC/TOF-MS results in a dramatic improvement in analyte separation and identification.15
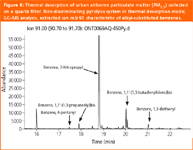
Figure 6: Thermal desorption of urban airborne particulate matter (PM2.5) collected on a quartz filter. Non-discriminating pyrolysis system in thermal desorption mode; GCâMS analysis, extracted ion m/z 91 characteristic of alkyl-substituted benzenes.
Figure 7 presents a two-dimensional chromatogram (2D contour plot) obtained for the extracted ion m/z 91 characteristic of alkylbenzenes by GC×GC/TOF-MS. Each spot represents an individual compound; the colour of each spot codes the abundance of that compound in the sample. Any compounds located above each other along the y-axis would co-elute in conventional GC. When a mixture is separated based on two independent mechanisms (as in GC×GC), each compound ends up in a unique location on the separation plane. Compounds belonging to the same chemical class are related to one another in some chemical or physical way, thus they usually form a distinct band on the 2D chromatogram. This feature facilitates the identification of unknown compounds. The main advantages of the non-discriminated pyrolysis system in this application include no sample preparation, the ability to perform both thermal desorption of the samples and their subsequent pyrolysis, as well as the proven ability to detect high-molecular weight compounds in the samples.
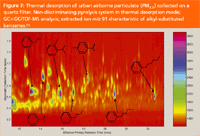
Figure 7: Thermal desorption of urban airborne particulate (PM2.5) collected on a quartz filter. Non-discriminating pyrolysis system in thermal desorption mode; GC3GC/TOF-MS analysis; extracted ion m/z 91 characteristic of alkyl-substituted benzenes.15
Conclusions
Non-discriminating pyrolysis extends the range of pyrolysis products that can be analysed by GC–MS to include high-boiling point compounds, which are normally lost on transfer from conventional pyrolysis units to the GC column. The system can be used for many different kinds of materials, both natural and artificial, and only a very small amount of sample is required. Apart from regular pyrolysis, the system can also be used for thermochemolysis, which is particularly useful when conventional pyrolysis leads to defunctionalization of the analytes. The non-discriminating pyrolysis unit can be coupled easily to any gas chromatograph, GC–MS or GC×GC–MS system. The last combination is particularly promising, as it offers tremendous separation power, which is critical considering the very complex nature of many pyrolysates.
Acknowledgements
This research was supported in part by the Natural Sciences and Engineering Research Council of Canada. Ewa Dabek-Zlotorzynska and Luyi Ding are gratefully acknowledged for the PM samples. Our thanks go to Restek for supplying the Silcosteel tubing, and to SGE and Varian for the columns used in the study.
Ziba Parsi obtained her BSc in Chemistry from the University of Tabriz, Iran. In 1999 she graduated from Seneca College, Toronto, Canada following a pharmaceutical chemical technology programme. Currently she is a graduate student at the Department of Chemistry, University of Waterloo, Canada, working on the development and applications of non-discriminating pyrolysis. Tadeusz Górecki is an associate professor at the Department of Chemistry, University of Waterloo, Canada. He obtained his MSc eng. (1981) and PhD (1986) degrees in chemistry from the Gdansk University of Technology, Poland. His scientific interests include analytical pyrolysis, environmental analysis and chromatographic techniques. Juergen Poerschmann is a research associate at the UFZ Centre for Environmental Research in Leipzig-Halle, Germany. He received his MSc (1974), PhD (1978) and DSc (1990) degrees in Chemistry from the University of Leipzig, Germany. His scientific interests include oxidation processes in groundwater/wastewater remediation and characterization of humic organic matter.
References
1. T. P. Warmler, J. Chromatogr. A, 842, 207 (1999).
2. William J. Irwin, "Analytical pyrolysis. A comprehensive Guide", Dekker, (1982).
3. J. Poerschmann et al., J. Microcolumn Sep., 10, 401, (1998).
4. D. M. White et al., J. Anal. Appl. Pyrol., 71, 107–118 (2004).
5. T. P. Wampler, J. Anal. Appl. Pyrol., 71, 1–12 (2004).
6. T. Górecki and J. Poerschmann, Anal. Chem., 73, 2012–2017 (2001).
7. Z. Parsi, T. Górecki and J. Poerschmann, J. Anal. Appl. Pyrol., 73, 89–96 (2005).
8. J. M. Challinor, J. Anal. Appl. Pyrol., 25, 349 (1993).
9. J. Harynuk and T. Górecki, J. Chromatogr. A, 1019, 53–63, (2003).
10. J. Poerschmann, T. Górecki and Z. Parsi, LabPlus International, Feb/March, 8–14 (2005).
11. I. K. Kögel-Knabner, Organic Geochemistry, 31, 609–625 (2000).
12. V. Gobé, L. Lemée and A. Amblès, Organic Geochemistry, 31, 409–419 (2000).
13. H. Schulten and P. Leinweber, J. Anal. Appl. Pyrol., 38, 1–53 (1998).
14. J. Poerschmann, Z. Parsi and T. Górecki, J. Chromatogr. A, 1071, 99–109 (2005).
15. Z. Parsi et al., Chemia i Inzynieria Ekologiczna (Poland), in print.

Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.
A Guide to (U)HPLC Column Selection for Protein Analysis
April 16th 2025Analytical scientists are faced with the task of finding the right column from an almost unmanageable range of products. This paper focuses on columns that enable protein analysis under native conditions through size exclusion, hydrophobic interaction, and ion exchange chromatography. It will highlight the different column characteristics—pore size, particle size, base matrices, column dimensions, ligands—and which questions will help decide which columns to use.












