Profiles in Practice: Advances in Science and Geopolitical Issues
LCGC Europe
The Centers for Disease Control and Prevention (CDC) estimates that each year in the United States, 76 million people get sick, 325000 are hospitalized and 5000 die from food-related illnesses. Food-borne illness is a serious public health problem. -National Library for the Environment, Food Safety Issues in the 107th Congress, 2001, Donna U. Vogt.
Regardless of whether analytical chemists focus their investigations on small molecules or life sciences, liquid chromatography–mass spectrometry (LC–MS) is now a staple technology in their industry. Nevertheless, few areas of LC–MS practice have been so affected by geopolitics as that of food safety analysis, a topic as important to us all as it is complex.

Table 1: This Monthôs Featured Scientists
Food safety assays are as well-characterized as any we encounter in any regulated practice. Yet, as we find with other areas of practice, the approach taken by the European Union (EU) differs from that taken by the US. Moreover, these disparities in practice are more significant in the area of food surveillance than in any other.
The Case for Surveillance
Consider, for example, the recent well-publicized difference in methodologies between the EU and US regarding vigilance for growth-promoting hormones and testing for bovine spongiform encephalopathy (BSE). The EU imposes a zero-level tolerance for administering growth promotors to cattle and poultry, and it tests far more of the meat entering the market than the US does. In 2001, a comparison showed that 1 US cow in 18000 intended for market was tested. But EU legislation requires that, as an absolute minimum, 1 cow in 250 must be tested. Non-EU members impose similar stringent testing requirements: in Switzerland, 1 cow in 60 was tested in 2001.
Since 1989, the EU has banned imports of meats produced with growth-promoting hormones. The US Department of Agriculture (USDA) estimated in 2001 that the EU ban costs the US about $200 million annually in lost exports, mainly of beef. The impact can be even greater on emerging nations that depend on food exports as a primary economic factor.
As a national reference laboratory for the UK, Northern Ireland's Veterinary Sciences Division (VSD) of the Department of Agriculture and Rural Development performs statutory veterinary drug residue testing for the province of Northern Ireland. In an address this year at the American Society of Mass Spectrometry (ASMS) meeting in San Antonio,1 Paul Young of the VSD stressed that antibiotic resistance is an important concern that arises from veterinary drug residues, and he explained why we shouldn't allow antibiotics into our food:
Clearly, if microorganisms are being exposed to these drugs on a daily basis, they may develop resistance to the antibiotics. And because most of the antibiotics used in veterinary medicine are the same as those your doctor prescribes for you, they could eventually lose their efficacy, causing a big problem for us all. Allergenicity is another concern. As much as 3% of the population may be allergic to penicillin. So to expose the whole population to these things on a daily basis is obviously unwise.
Antibiotics find their way into our food supply in several ways. Notably, failure to observe prescribed withdrawal periods for permitted drugs can cause higher-than-allowable concentrations when an animal is slaughtered.
Another common route of veterinary antibiotics into the human food supply stems from the practice of administering illegal and unlicensed antibiotics as a prophylaxis against disease organisms in food-producing animals. Producers find this easy to do because the drugs are readily obtainable and cheap. Thus, they serve as cost-effective insurance against possible loss from illness. Moreover, dramatic profit margins can make this practice pronounced, as in the case of aquaculture. Young relates, "When in Southeast Asia a few years ago, I was told that a shrimp farmer there can afford to lose one crop out of every three and still make quite a big profit. But if he uses these drugs as a general prophylaxis against disease, he may not even need to lose one crop."
Finally, some antibiotics also promote growth. So the double benefit of better animal health and markedly increased weight gain on less feed is a compelling incentive to use the drugs.
Hans van Rhijn of RIKILT—Institute of Food Safety, which is the Netherlands' national reference laboratory for veterinary drugs, has in recent years seen significant technological advances in his practice, the result of LC–MS increasing the quality of analytical interrogation. Interest in residue analysis — especially in animal produce — in the last five years or so has increased dramatically. RIKILT employs 180 people, of whom about 50% are involved in residue analysis or regulatory issues regarding residues. It performs a broad range of analytical tasks that include monitoring for legal and illegal veterinary drugs and detecting dioxins, polycyclic aromatic hydrocarbons, marine toxins and growth-promoting hormones (whose use is illegal in the EU). In particular, RIKILT tests for antibiotics such as nitrofuran, whose carcinogenic and mutagenic effects when ingested present a health risk to humans. Though the parent compound is excreted quickly, it nevertheless produces a reactive intermediate that binds readily to protein and must be assessed in food products as a tissue-bound residue.
The practice of administering nitrofuran drugs to food-producing animals had been banned because the drugs were suspected of inducing carcinogenic residues in animal tissues. Enforcing such a ban requires close monitoring of abuse, one of the main tasks of numerous laboratories throughout Europe.
Although nitrofurans were the major problem in the period February 2002–2004, in September 2001, testing had previously discovered chloramphenicol residues in Chinese, Vietnamese and Indonesian shrimp. Chloramphenicol is a potent, broad-spectrum antibiotic drug, banned for use in food-producing animals in the EU since 1994.
Chloramphenicol was so prevalent in Chinese products — honey, rabbit meat, poultry, and crustaceans such as shrimps and prawns — that a ban on further imports to EU markets was imposed in January 2002. On June 14, 2002, the FDA announced it would follow the EU's lead and increase vigilance.2 The initial detection of chloramphenicol, together with previously uncovered poor hygiene and the virtual absence of food safety control programmes in the countries under investigation3 triggered the import ban and led to further investigation. Both the EU and US voiced concern about the antibiotic's residues in shrimp products. They began strict inspections for chemical residues in all shrimp imported from Asian countries.
Advances in Analytical Chemistry Prompt Difficult Far-Reaching Decisions
Young tells about testing tiger shrimp imports from Thailand for nitrofuran and finding that 21% contained the drugs at measurable concentrations. Van Rhijn discovered the problem of drugs in aquaculture and introduced the issue in 2002 with often-cited work that effectively illustrates the concern.
On February 7, 2002 RIKILT reported the first finding of nitrofuran residues to the EU's Rapid Alert System for Food and Feed (RASFF), an action that triggered a close examination of the food supply for similar potential hazards to public health. (It's worthwhile to mention RASFF's website: http://europa.eu.int/comm/food/food/rapidalert/index_en.htm. The system offers an effective means of alerting those concerned with food safety, both within and outside the EU).
Between February and April of 2002, 60% of the imported shrimp samples tested in van Rhijn's laboratory proved positive for banned substances, in particular, tissue-bound residues of nitrofurans. The goal then became to provide 100% testing for residues, because the risk and prospect for occurrence was great.
Beginning in March 2002, the EU ban against Chinese food products was put in place. Only a month later, because of the high residue incidence in SE-Asian shrimp, the EU prompted a 100% inspection of imported poultry and shrimp from Thailand, Vietnam, and Myanmar and, in October 2002, 100% testing of Brazilian poultry.4 (The bans have since been relaxed and normal testing and inspection protocols resumed, the result of success in both Thai and Brazilian testing programmes.)
The Not-So-Level Playing Field
Here the problem becomes knotty. As van Rhijn puts it, the emerging nations whose economies depend upon exports might not be able to test to the same level of proficiency that established nations can and do. His laboratory, and others like it, can test with greatly increased sensitivity for the presence of banned compounds using today's LC–MS instruments. Indeed, they can perhaps measure levels present beyond the level of toxicological relevance in humans. Van Rhijn says, "We can work at 0.1 ppb where, a few years ago, 1 ppb might have been the reference limit."
A parameter introduced in EU legislation in 2002 (CD 2002/657/EC), the minimum required performance limit (MRPL), was not originally intended as an action limit but rather a minimum requirement for a laboratory's ability to test for a given substance. Though the MRPL was conceived as a harmonization tool, it failed in that regard. Indeed, it achieved quite the opposite: any concentration between zero — actually CC-alfa — and the MRPL was regarded a violation.
Under Commission Decision 2002/657/EC (article 6.1), any finding above CC-alfa is a violation. But CC-alfa often is unrealistically low, lower than virtually every nonzero finding. So a finding confirmed according to established identification criteria almost invariably constitutes a violation. CC-alfa is a laboratory-specific or method-specific performance characteristic. Its numerical value, then, is rather subjective and certainly not harmonized, differing from one laboratory to another depending upon expertise, technical equipment, etc. It is because of this difference in the numerical value of CC-alpha that a violation in one country might not be detected in another. The combined effect of our ability to test for compounds at extremely low concentrations and the EU's zero-tolerance policy, which declares any detectable residue on its "A" list a violation — the "A" list includes growth hormones and banned antibiotics — creates a not-so-level playing field. As van Rhijn points out, an importing country's ability to perform sensitive testing can result in the banning of a product in one country and not in another.
However, recently, Commission Decision 2005/34/EC adopted the MRPL as the action limit for imported consignments, hence today's discussion about the significance of zero-tolerance limits.
Thailand, the world's largest producer of farm-raised shrimp, accounts for 33% of global shrimp aquaculture. In 2001, it was the world's largest shrimp exporter. But increased inspections of shrimp product exports created a decline that started in 2001 and continued until 2002.
Equipping the Thai government and private laboratories with state-of-the-art LC–MS-MS instrumentation played a key role in correcting the problem together with training programmes organized by RIKILT for EU and other laboratory workers. Glenn Kennedy and Paul Young, both of Northern Ireland's VSD, organized and supplied on-the-spot training to the Thai government. Kennedy initiated the FoodBRAND project in 1999, which developed modern methods for detecting nitrofurans, methods that proved critical to helping governments, including Thailand, Brazil and Ecuador refine their practice.
Young says that, in 2002, Thailand had "zero LC–MS instruments — not one." He goes on:
And yet, in about nine months, the Thais installed instruments and trained operators who worked around the clock. In one laboratory, people performed nitrofuran analyses in three shifts, 24 hours a day, seven days a week. Their initial efforts were aimed at eliminating nitrofuran and chloramphenicol use, but only in produce destined for export. It was dollars and cents. 'Let's safeguard our market,' they reasoned, and so they tested only produce that would be exported — and they tested 100% of it.
Today, the Thais are expanding their testing to include products used in domestic consumption. Brazil, China and others did the same. But the testing requirements are staggering — billions of pounds of chicken, pork and beef require monitoring. Moreover, this speaks nothing of the difficulty of expanding the scope of easily taught accessible tests, such as the enzyme-linked immunosorbent assay, to effectively detect a wide range of structurally dissimilar compounds simultaneously.
Analytical chemists continue to develop instrument skills and testing protocols. Since about 1999, LC–UV, the traditional analytical method has been augmented increasingly and replaced in routine residue laboratory work by LC–MS-MS. This is because achieving 1 ppb via UV detection for most compounds present in biological matrices can be difficult. Furthermore, confirmation of the analyte's identity is obligatory for banned as well as authorized compounds (for the latter when the maximum residue limit or MRL is exceeded). For banned substances especially, MS is the preferred confirmatory technique.
Technologia ex Machina
Because it is useful to revisit results as new standards evolve, van Rhijn began to use time-of-flight (TOF) mass spectrometers.
Such work, which began only recently, is encouraging. Analysing milk samples, van Rhijn finds he can detect and quantify over 25 compounds representing six chemical classes of veterinary drugs at their MRLs — prepared using a generic technique all in a single, 6 min analytical run.5
Ramesh Rao, Business Manager for Industrial Mass Spectrometry at Waters, points to the trend from traditional compound class-specific methods to multiresidue methods with improved limits of detection and increased peak capacity (the number of discrete peaks detected in a given amount of chromatographic space6 ).
As legislation evolves, it must do so in keeping with technological capabilities. Rao described the effort of the Japanese to overhaul their legislation and bring it in line with EU standards as an example of the current trend to incorporate increased technological capability:7
Particular contaminants, which come to light on testing of domestic or imported produce, and which are reported in the press, are most often of higher concentration than that allowed during routine surveillance. Unexpected compounds, however, also are detected. These do not fall under routine testing regimens because no legislation currently targets them.
Recent examples of these unexpected compounds are mycotoxins, found in nuts, rice and spices; marine biotoxins, found in fish and shellfish; Sudan red, found in chilli powder, Worcestershire sauce and other condiments. (Sudan red is an industrial dye used for coloring solvents, oils, waxes and shoe and floor polishes.)
Young, van Rhijn, and others have kept abreast of testing requirements by upgrading to more sophisticated instruments that provide increased signal-to-noise ratio and faster transition times for discrete ions. Young gives an example of the nature of his analytical task:
I'm looking for the presence of 22 compounds that we can confirm in one injection. We monitor 53 transition ions using a dwell time of 10 milliseconds and achieve 48 data points across the peak. So, clearly, there is significant scope to increase the number of transitions, the number of drugs we're looking for.
EU legislation mandates a number of criteria for LC methods. For example, the analyte of interest's retention time must be more than twice the void volume of the system, and relative retention times must correspond within 2.5%. Novel technologies like ultrahigh pressure chromatography, based upon Professor James Jorgenson's work at the University of North Carolina and commercialized by Waters as Ultra Performance LC (UPLC), deliver peak widths at 0.05 min and attract interest for good reason. Once employed, they become critical to success. Young's evaluation shows critical requirements with measurement time windows spanning as little as six seconds each. According to him, "It is very important that these peaks don't drift. If they drift by more than a couple of seconds, either way, I run a very significant risk of missing the peak."
Figure 1, from a recent presentation by Young, shows the repeatability of retention time by overlaying the results of 16 injections spread over eight hours. Young's laboratory can now confirm the presence of 22 growth hormone compounds by monitoring at least two transition ions. (EU regulations require a confirmatory MS method that demonstrates four ions with three ion ratios [single MS] or two daughter transition products [MS-MS]). However, using a UPLC method, separation is achieved in only 4 min. Also, quite crucially, he notes, "The first peak is eluted at 2.4 min, thus meeting the EU criteria for chromatographic retention. So not only can we separate all the drugs, confirm them all in one injection, but we can do it in less time."
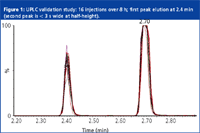
Figure 1: UPLC validation study: 16 injections over 8 h; first peak elution at 2.4 min (second peak is
Where does the conventional wisdom believe food safety analysis is headed in the future? A clear indication, based upon the growing demand for MS as pesticide maximum residue limits (MRL) decrease, can be gleaned from an article in the 30 June, 2005 edition of the newsletter "Instrument Business Outlook," published by Strategic Directions International, Inc. (SDI). The article states, in part, that:
The Food and Beverage industry is experiencing pressure from both governmental institutions and consumers to find new ways for detecting and quantifying contaminants in food and beverages. These new regulations are driving growth for analytical instrumentation for laboratory and process environments, as more efficient analysis techniques with higher sensitivity are needed. In 2004, analytical instrument sales to the food and beverage industry totalled about $1.28 billion and are forecast to grow about 5% over the next five years. The fastest growing category of instrumentation technology for this industry has been atomic spectroscopy. However, this sales growth is expected to slow and be replaced as the fastest growing category of instruments by mass spectrometry.
"MS in Practice" editor Michael P. Balogh is principal scientist, LC–MS technology development at Waters Corp. (Milford, Massachusetts, USA.); an adjunct professor and visiting scientist at Roger Williams University (Bristol, Rhode Island, USA); and a member of LCGC Europe's Editorial Advisory Board. Direct correspondence about this column to "MS in Practice", LCGC Europe, Advanstar House, Park West, Sealand Road, Chester CH1 4RN, UK, or e-mail: dhills@advanstar.com
References
1 P. Young, 52nd ASMS, San Antonio, Texas, June 2005.
2 http://www.fda.gov/bbs/topics/NEWS/2002/NEW00815.html.
3 For details refer to the China report November 2001 of the Food and Veterinary Office at http://europa.eu.int/comm/food/fs/inspections/vi/reports/index_en.html.
4 http://www.foodqualitynews.com/news/news-ng.asp?id=12950-uk-supports-tests.
5 H. van Rhijn, 52nd ASMS San Antonio, Texas, June 2005.
6 J. Castro-Perez, R. Plumb, J.H. Granger, I. Beattie, K. Joncour, and A. Wright, Rapid Commun. Mass. Spectrom. 19(6), 843–848 (2005).
7 Robust and Reliable, Food Safety Europe 2005, (GDS Publishing, Inc., New York, New York).

Determining the Link Between Prenatal Cannabis Use and Symptoms of Depression Using LC–MS/MS
April 16th 2025Researchers investigating the relationship between cannabis use during pregnancy and depressive symptoms—and whether continued use beyond the first trimester or higher levels of use were linked to increased symptoms—used liquid chromatography–tandem mass spectrometry (LC–MS/MS) to confirm the presence of 11-nor-9-carboxy-delta-9-tetrahydrocannabinol (THC-COOH) in urine samples.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.












