Next-Generation Autosampler for LC–MS Equipped with Straight Injection TechnologyTM
This article reports a new sample injection mechanism for an autosampler that directly transfers a sample to column without passing it through any flow-changing device, and hence minimizes a delay volume and reduces a carryover signal to the extreme level.
Yoshikazu Sugito, Tsuneaki Kaneko, and Osamu Shirota, SHISEIDO Co., Ltd.
This article reports a new sample injection mechanism for an autosampler that directly transfers a sample to column without passing it through any flow-changing device, and hence minimizes a delay volume and reduces a carryover signal to the extreme level.
Carryover is one of the most common problems one often encounters in wide dynamic-range measurements with a high-end mass spectrometer. There are various sources leading to the carryover, such as adsorption of low-solubility compounds on column and instrumental surfaces, especially in gradient elution. In many cases, an autosampler seems to be the most responsible factor to the problem. Although it has been largely improved, anti-carryover performance in current HPLCs may not be adequate to fully exploit the sensitivity of high-end mass spectrometers. This article describes a new autosampler having straight injection technology (SIT), which can drastically reduce carryover in LC–MS.
Experimental
Two autosamplers, with and without SIT, were used for the comparative study. Carryover values of reserpine were measured under isocratic conditions, and those of chlorhexidine were measured under gradient conditions. An API5000™ mass spectrometer (AB SCIEX) and a UV detector were used as detectors.
Results
The newly-designed autosampler did not show any carryover of reserpine (Figure 1) in an isocratic elution even after introduction of overloading quantity. For chlorhexidin, a carryover peak of magnitude one tenth that of the conventional model (Figure 2) was slightly observed under gradient conditions. These results indicated that the direct transfer of samples to a column greatly contributed to the reduction of carryover.
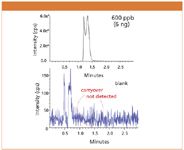
Figure 1: Carryover of reserpine under isocratic conditions. Column: CAPCELL PAK C18 MGII S3 2.0 mm i.d. x 50 mm, Mobile phase: A) 0.1 vol % HCOOH, B) CH3CN, A/B = 60/40, Flow rate: 0.2 mL/min, Temperature: 40 °C, Detection: ESI Positive (API5000â¢).
The simplified sample transfer pathway from a sample-collection needle to a separation column also contributed to reproducibility of peak area and retention time, number of theoretical plates, and resolution between peaks. The ultimately reduced delay volume and shortened pathway seem to have reduced sample diffusion to a large extent. Using stainless steel-reinforced polyetheretherketone (PEEK) tubing, the autosampler could tolerate 70 MPa and must be the most pressure-resistant among "inert" HPLCs.
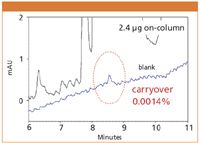
Figure 2: Carryover test of chlorhexidine under gradient conditions. Column: CAPCELL PAK C18 MGIII S5 2.0 mm i.d. x 150 mm, Mobile phase: A) 10 mmol/L KH2PO4, 100 mmol/L NaClO4 (pH = 2.6), B) CH3CN, B: 30% (0.0min) â 70% (10.0 min) gradient, Flow rate: 0.2 mL/min, Temperature: 40 °C, Detection: UV 260 nm.
Conclusion
The newly developed injection mechanism improved separation efficiency and precision in HPLC and LC–MS, including those obtained under gradient conditions.

SHISEIDO Co., Ltd.
1-1-16 Higashi-shimbashi, Minato-ku, Tokyo 105-0021, Japan
tel. + 81-3-6253-1412; fax + 81-3-6253-1416
Email: hplc@to.shiseido.co.jp; Website: www.shiseido.co.jp

The Benefits of Custom Bonded Silica
April 1st 2025Not all chromatography resins are created equal. Off-the-shelf chromatography resins might not always meet the rigorous purification requirements of biopharmaceutical manufacturing. Custom bonded silica from Grace can address a wide range of separation challenges, leading to real performance improvements. Discover more about the latest innovations in chromatography silica from Grace, including VYDAC® and DAVISIL®.
5 Things to Consider When Selecting a Chromatography Silica
April 1st 2025Particularly in the pharmaceutical industry, drug purity isn’t just a goal – it’s essential for achieving safety, stability and efficacy. However, purification is easier said than done, especially with challenging molecules like DNA and RNA “oligonucleotides,” due in large part to their diversity and the range of impurities that can be generated during production. Enter DAVISIL® chromatographic silica, with a wide range of pore diameters and particle sizes to meet your specific application, performance and sustainability requirements. Before you choose the chromatography resin for your next purification application, take a look at these 5 considerations.
Automating Protein Purification: Efficiency, Yield, and Reproducibility
March 27th 2025Recent advancements in automated protein purification stress the importance of efficiency, scalability, and yield consistency. This eBook compares different purification platforms, highlighting their impact on downstream applications and demonstrating how automation enhances throughput and process control.
MilliporeSigma: Ultrapure Water for Sensitive LC-MS Analysis of Pesticides
March 25th 2025The aim of the study was to illustrate the efficiency of Milli-Q® water purification systems in eliminating pesticides from tap water, thereby producing and delivering reliable and consistent-quality ultrapure water suitable for pesticides analysis















