Confirmation and Quantitation of Cocaine and Major Metabolites in Urine Using the ISQ Single Quadrupole GC–MS
Cocaine is a central nervous system stimulant derived from Erythroxylon coca.
Matthew Lambing, Eric Phillips, and Trisa Robarge, Thermo Fisher Scientific
Cocaine is a central nervous system stimulant derived from Erythroxylon coca. It is metabolized in-vivo resulting in the formation of ecgonine methyl ester, norcocaine, and benzoylecgonine. Cocaethylene is a substance formed when cocaine and ethanol are coadministered (1). A forensic toxicology method for the confirmation and quantitation of ecgonine methyl ester (EME), benzoylecgonine (BE), cocaine (COC), and cocaethylene (CE) in human urine was developed using the Thermo Scientific ISQ single quadrupole GC–MS system.
Methods
Each sample batch contained a matrix-matched single point calibrator (at 150 ng/mL), quality control samples set to contain each target compound at 40% and 125% of the calibrator (60 ng/mL and 187.5 ng/mL respectively), and a negative control (blank urine with internal standard only). Thermo Scientific HyperSep Verify-CX solid phase extraction columns were used for sample extraction. Samples were derivatized with hexafluoroisopropanol (HFIP) and pentafluoropropionic acid (PFPA or PFAA).
The ISQ™ mass spectrometer system was operated in selected ion monitoring mode, collecting 3 ions for each target compound, and 2 ions for each deuterated internal standard (Table I). A Thermo Scientific AS 3000 II autosampler and a Thermo Scientific TRACE GC Ultra gas chromatograph, equipped with a split/splitless injection port, provided sample introduction and separation. A 15 m x 0.25 mm i.d. x 0.25 µm film thickness Thermo Scientific TraceGOLD TG-5MS analytical column was used to enhance separation of the target cocaine class compounds from each other and from matrix components. Thermo Scientific ToxLab Forms software automated the acquisition and processing of all data, including quantitation and ion ratio confirmation calculations. For precision analyses, a coefficient of variation of <10% of the average calculated quality control amounts were required for each analyte, and inter-day percent differences of calculated amounts also had to be less than 10%.
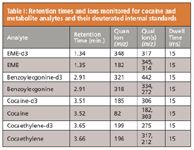
Table I: Retention times and ions monitored for cocaine and metabolite analytes and their deuterated internal standards
Results
- Assay linearity ranged from 15 ng/mL to 12,500 ng/mL for BE, EME, and CE, and 15 ng/mL to 5,000 ng/mL for cocaine.
- Limits of detection and quantitation of 15 ng/mL using a 2 mL sample size.
- Intra- and inter- day precision of <10% CV at the quality control levels of 60 ng/mL and 187.5 ng/mL.
- Correlation coefficient (R2 ) better than 0.9990 for cocaine, benzoylecgonine, ecgonine methyl ester, and cocaethylene based on a one point calibration.
- Pseudoephedrine at a concentration of 20,000 ng/mL showed interference with EME at the 40% and 125% QC levels.
- Norcocaine at 10,000 ng/mL demonstrated no interference with any analyte tested, but limited co-elution was observed with cocaethylene. Relative retention time to CE = 1.005.
Conclusion
A method was developed to demonstrate the performance of the ISQ GC–MS system for the confirmation and quantification of cocaine and its major metabolites in a urine matrix. The assay described offers broad linearity to cover a wide range of analyte concentrations, thus, reducing the need for dilutions or repeat extractions. Excellent precision was also demonstrated around the 150 ng/mL cutoff, with CV measurements of 10% or less over the study. Limits of detection and quantification at 15 ng/mL ensure sensitive performance for retest and directed assay samples. The methodology described offers a means for a forensic toxicology laboratory to confirm and quantify cocaine, benzoylecgonine, ecgonine methyl ester, and cocaethylene in human urine.
References
(1) Disposition of Toxic Drugs and Chemicals in Man, Eighth Edition. Randall C. Baselt, Biomedical Publications, 2008.
All trademarks are the property of Thermo Fisher Scientific Inc. and its subsidiaries.

Thermo Fisher Scientific
2215 Grand Avenue Parkway, Austin, TX 78728
tel. (800) 532-4752; fax (561) 688-8731
Website: www.thermoscientific.com

The Benefits of Custom Bonded Silica
April 1st 2025Not all chromatography resins are created equal. Off-the-shelf chromatography resins might not always meet the rigorous purification requirements of biopharmaceutical manufacturing. Custom bonded silica from Grace can address a wide range of separation challenges, leading to real performance improvements. Discover more about the latest innovations in chromatography silica from Grace, including VYDAC® and DAVISIL®.
5 Things to Consider When Selecting a Chromatography Silica
April 1st 2025Particularly in the pharmaceutical industry, drug purity isn’t just a goal – it’s essential for achieving safety, stability and efficacy. However, purification is easier said than done, especially with challenging molecules like DNA and RNA “oligonucleotides,” due in large part to their diversity and the range of impurities that can be generated during production. Enter DAVISIL® chromatographic silica, with a wide range of pore diameters and particle sizes to meet your specific application, performance and sustainability requirements. Before you choose the chromatography resin for your next purification application, take a look at these 5 considerations.
Automating Protein Purification: Efficiency, Yield, and Reproducibility
March 27th 2025Recent advancements in automated protein purification stress the importance of efficiency, scalability, and yield consistency. This eBook compares different purification platforms, highlighting their impact on downstream applications and demonstrating how automation enhances throughput and process control.
MilliporeSigma: Ultrapure Water for Sensitive LC-MS Analysis of Pesticides
March 25th 2025The aim of the study was to illustrate the efficiency of Milli-Q® water purification systems in eliminating pesticides from tap water, thereby producing and delivering reliable and consistent-quality ultrapure water suitable for pesticides analysis















