Determination of Furan in Food by Gas Chromatography– Mass Spectrometry and Headspace Sampling
Furan is naturally occurring at low levels in many foods and drinks (1).
Padmaja Prabhu, PerkinElmer, Inc.
Furan is naturally occurring at low levels in many foods and drinks (1). Based on studies in laboratory animals, furan consumption has been classified by IARC as possibly carcinogenic to humans. The primary source of furan in food is considered to be thermal degradation of carbohydrates, such as glucose, lactose, and fructose.
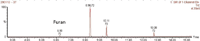
Figure 1: Chromatogram furan peak in coffee sample.
This application note demonstrates a rapid method for the identification and quantification of furan in food samples using gas chromatography (GC), headspace sampling (HS), and mass spectrometry (MS). In addition to method optimization and standard analysis we have analyzed a number of food samples for furan.
The PerkinElmer® Clarus® 680 Gas Chromatograph, Clarus 600 C Mass Spectrometer, and a TurboMatrix™ HS-40 system was used for this application. The separation was achieved on an Elite™-624 column ramped from 40 °C to 250 °C over 20 min. The mass spectrometer was scanned from m/z 35–150 with 5 scans per s.
Headspace was chosen as the sample introduction technique because furan can easily be partitioned from liquid matrices, and headspace facilitates automated extraction of furan without the instrument contacting the sample matrix. Caution must be taken when setting the vial oven temperature; a high temperature can result in furan formation in the sample during analysis. To reduce this risk, the method presented here uses a temperature of 60 °C.
The GC–MS was calibrated across the range of 1.0 to 40 ng/mL, each calibration point was run in triplicate to demonstrate the precision of the system, the average %RSD across the range was 4.3%. The coefficient of determination for a line of linear regression across the range of 1.0 to 40 ng/mL was 0.9997.
Sample Preparation
Samples were collected from the local food market. These samples included coffee, milk, canned foods, sauces, peanut butter, and apple juice. 10 mL of sample was transferred into a headspace vial; 4 g of NaCl was added to it. Milk and other viscous samples were diluted with water (1:2 or 1:4). The semi-solid samples were ground and 5 g of sample was added to the headspace vials with 5 mL of saturated salt (NaCl) solution. Coffee powder was dissolved following directions on the package, and then treated like a nonviscous liquid sample.
The recovery of the method was tested with the analysis of the brewed coffee sample spiked at three different levels: 2, 5, and 10 µg/L. The measured amount was 2.03, 5.44, and 9.54 µg/L demonstrating the technique to be quantitative.
Results
Eight samples of common beverages were analyzed using the HS-GC–MS method developed here. Brewed coffee was demonstrated to have the highest levels of furan at 250 µg/L.
Conclusion
This application provides a method for the determination of furan in beverages using headspace sample introduction. Headspace-GC is fast, reliable, and can be used for the quantification of furans in common beverages. The internal standard calibration of furan across 1–40 µg/L fit responded linearly. Beverages were analyzed and the level of furan was determined. Furan was identified by both the retention time and the MS fragmentation pattern. This method was validated at several levels on coffee matrix recovery, with values between 95–101%.
PerkinElmer, Inc.
940 Winter Street, Waltham, MA 02451
tel. (800) 762-4000; fax (203) 944-4904
Website: www.perkinelmer.com


Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.

















