A New High-Resolution Size Exclusion Chromatography Column for MAb Aggregate Analysis
The new MAbPacTM size exclusion (SEC) column is based on high-purity, spherical, porous (300 Å), 5 µm silica covalently modified with a proprietary diol hydrophilic layer.
Srinivasa Rao, Xiaodong Liu, and Chris Pohl, Dionex Corporation
The new MAbPac™ size exclusion (SEC) column is based on high-purity, spherical, porous (300 Å), 5 μm silica covalently modified with a proprietary diol hydrophilic layer. The column is packed into nonmetallic, biocompatible, PEEK™ column housing to eliminate compromised chromatography due to the presence of metal from the column hardware. This offering results in minimal nondesired interactions between the biomolecules and the stationary phase. Stable surface bonding leads to low column bleed and enhances compatibility with MS, ELSD, and Corona® CAD® detection. The column is available as 4 × 300 mm analytical and 4 × 50 guard column formats.
Experimental
A Dionex ICS 3000 HPLC system with a DP pump, VWD absorbance detector, auto-sampler, and a TC thermostatted column compartment was used.
Chromatography was controlled by Chromeleon® Chromatography Data System software (Dionex).
Monoclonal antibody was a gift from a local biotech company. Other analytical grade chemicals used in the buffers were obtained from Sigma. Super SW3000 SEC column was obtained from Tosoh Bioscience.
Results and Discussion
MAbPac SEC-1 is a size exclusion chromatography (SEC) column specifically developed for characterization and analysis of monoclonal antibody (MAb) aggregates, papain, or other enzyme digested fragments and for other size based separation applications (1). The diol stationary phase is designed to minimize undesired interactions to handle both high and low salt mobile phase eluents and mass spectrometry compatible volatile eluents.
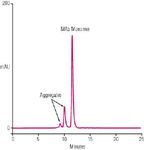
Figure 1: MAb aggregate analysis using MAbPac SEC-1 column. Mobile Phase: 300 mM NaCl in 50 mM phosphate buffer (pH 6.8); Flow Rate = 0.2 mL/min; Sample = MAb 10 mg/mL; Injection volume= 2 μL; detection; UV at 280 nm; Temperature = 30 °C.
Size-exclusion chromatography (SEC) is a well-accepted technique for the detection and accurate quantification of protein aggregates in MAbs and other biological drug products. An example separation of MAb aggregation using MAbPac SEC-1 is shown in Figure 1. A comparison of MAb analysis on MAbPac SEC-1 and Super SW3000 using mass spectrometry compatible mobile phase (100 mM ammonium acetate) is shown in Figure 2. The MAbPac SEC-1 column provided superior efficiency, peak shape and asymmetry, sensitivity, and better recovery when compared with Tosoh's Super SW3000 SEC column.
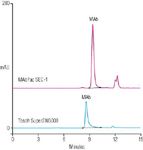
Figure 2: Comparison of MAbPac SEC-1 with Tosoh's Super SW 3000 using volatile eluents. Mobile Phase: 100 mM NH4OAC (pH 5.0); Sample = MAb 1 mg/ mL; Flow Rate = 0.25 mL/min (Dionex); 0.33 mL/min (Tosoh); Injection volume = 2 μL (Dionex); 2.5 μL (Tosoh); detection: UV at 280 nm; Temperature = 30 °C.
References
(1) MAbPac SEC-1 manual and data sheets are available at www.dionex.com
PEEK is a trademark of Victrex PLC.
CAD, Chromeleon, and Corona are registered trademarks, and MAbPac is a trademark of Dionex Corporation.
Dionex Corporation
1228 Titan Way, P.O. Box 3603, Sunnyvale, CA 94088
tel. (408) 737-0700; fax (408) 730-9403
Website: www.dionex.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.















